WO2011107855A2 - Sustained release oral liquid suspension dosage form - Google Patents
Sustained release oral liquid suspension dosage form Download PDFInfo
- Publication number
- WO2011107855A2 WO2011107855A2 PCT/IB2011/000426 IB2011000426W WO2011107855A2 WO 2011107855 A2 WO2011107855 A2 WO 2011107855A2 IB 2011000426 W IB2011000426 W IB 2011000426W WO 2011107855 A2 WO2011107855 A2 WO 2011107855A2
- Authority
- WO
- WIPO (PCT)
- Prior art keywords
- sustained release
- pellets
- dosage form
- drug
- ready
- Prior art date
Links
Classifications
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K31/00—Medicinal preparations containing organic active ingredients
- A61K31/33—Heterocyclic compounds
- A61K31/395—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins
- A61K31/40—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil
- A61K31/4015—Heterocyclic compounds having nitrogen as a ring hetero atom, e.g. guanethidine or rifamycins having five-membered rings with one nitrogen as the only ring hetero atom, e.g. sulpiride, succinimide, tolmetin, buflomedil having oxo groups directly attached to the heterocyclic ring, e.g. piracetam, ethosuximide
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/0087—Galenical forms not covered by A61K9/02 - A61K9/7023
- A61K9/0095—Drinks; Beverages; Syrups; Compositions for reconstitution thereof, e.g. powders or tablets to be dispersed in a glass of water; Veterinary drenches
-
- A—HUMAN NECESSITIES
- A61—MEDICAL OR VETERINARY SCIENCE; HYGIENE
- A61K—PREPARATIONS FOR MEDICAL, DENTAL OR TOILETRY PURPOSES
- A61K9/00—Medicinal preparations characterised by special physical form
- A61K9/48—Preparations in capsules, e.g. of gelatin, of chocolate
- A61K9/50—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals
- A61K9/5073—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings
- A61K9/5078—Microcapsules having a gas, liquid or semi-solid filling; Solid microparticles or pellets surrounded by a distinct coating layer, e.g. coated microspheres, coated drug crystals having two or more different coatings optionally including drug-containing subcoatings with drug-free core
Definitions
- the present invention relates to a stable, sustained release oral liquid suspension dosage form of pharmaceutical active ingredients, which is easy to administer and particularly beneficial for the pediatric and geriatric patients.
- This invention particularly relates to a sustained release oral liquid suspension dosage form which is a ready-to-use and does not require reconstitution before oral administration.
- Present invention also relates to a process for preparing said dosage form.
- Oral route is the most convenient and preferred route for the drug administration and there are various conventional dosage forms available for the oral administration of the drug such as tablets, capsules, solutions, emulsions or suspensions.
- solid oral dosage formulations especially large tablets or capsules are very difficult to swallow, hence for such patients liquid formulations are most preferred dosage forms and patients may be more inclined to comply with their medication instructions if the dosages are easier to ingest.
- sustained release solid dosage forms are available in the market such as extended release tablets of Levetiracetam (Keppra®), Metformin (Fortamet®), Metoprolol (Toprol-XL®), Desvenlafaxine (Pristiq®), and Venlafaxine (Effexor XR®).
- controlled release solid formulations requires large amounts of excipients which can retard the drug release in the formulation such as polymers, waxes as matrixing agents and increases the size of the final formulation which reduces patient compliance especially for the pediatric and geriatric patients. Therefore an alternative approach to administer sustained release dosage form will be an oral liquid dosage form.
- the suspension formulation against the solution can provide sustained drug release by controlling the drug release.
- Such formulations are particularly suitable for pediatric, geriatric patients and for those who can not swallow the tablet dosage form.
- the major challenge with suspension of sustained release dosage form would be to maintain the stability of suspension throughout the shelf life because in case of failure, irregular blood concentration may be achieved and can be sometimes sub therapeutic or toxic.
- the inventors of the present inventions have discovered a stable, taste masked oral sustained release dosage form.
- US5296236 discloses liquid- controlled release suspension of the drug containing micro granules coated with series of successive polymer having alternate hydrophobic and hydrophilic coatings.
- US 4999189 disclose sustained release oral suspensions wherein drug- resin particle coated with a first inner coating of water-insoluble wax and a second outer coating of a pharmaceutically acceptable water-insoluble polymer.
- WO2006093838 discloses method of preparing controlled release suspension of drug microbeads in thixotropic solution.
- US5156842 discloses oral sustained release suspension of the antibiotics, the antibiotic of said microparticles being coated with, distributed through or adsorbed onto the non-toxic polymer and finally such coated microparticles are suspended in of non-aqueous vehicles.
- EP0717992 discloses aqueous pharmaceutical suspensions containing a high amount of acetaminophen embedded in a polymer to impart controlled release properties.
- US20091 1 1872 discloses stabilized pharmaceutical suspension of carisbamate using hypromellose.
- US20030108575 mentions oral suspension formulation for the drug of low water solubility using wetting agent, thixotropic thickening agent and suspending agent.
- WO2003053403 discloses use of water-soluble or swellable nonsurfactant polymer along with the suspending agents for the poorly water soluble drug for the suspension formulation.
- US7300670 discloses an aqueous suspension of the particulate drug containing suspending agent in a liquid phase.
- WO2008122993 discloses drug containing microparticles with first pH independent polymer coating and second pH dependent polymer coating layer.
- US 6156340 disclose drug liquid sprayed on to inert core and such drug coated particles were further coated with two layers of the polymer having increasing permeability for the water.
- the present invention relates to a stable, sustained release oral liquid suspension dosage form of pharmaceutical active ingredients.
- the present invention is to provide a sustained release oral liquid suspension dosage form of pharmaceutically active ingredients, which is ready to use and does not require reconstitution before oral administration.
- An another aspect of the present invention is to provide a- sustained release oral liquid suspension dosage form of active ingredients which can be administered as once-daily or twice-daily.
- Another aspect of the present invention is to provide a sustained release oral liquid suspension dosage form of high solubility active ingredients, which can be administered as once-daily or twice-daily.
- Another aspect of the present invention is to provide a sustained release oral liquid suspension dosage form of active ingredients, which is stable during storage period and does not leach out in suspending media during storage period.
- Further aspect of the present invention is to provide a sustained release oral liquid suspension dosage form of active ingredients, which is particularly convenient for the pediatric and geriatric patient population.
- Another aspect of the present invention is to provide a process for the preparation of sustained release oral liquid suspension dosage form of active ingredients.
- Another aspect of the present invention is to provide a sustained release liquid suspension dosage form of active ingredients which comprises of the pharmaceutically acceptable additives like that of fillers, binders, lubricants, glidants, rate controlling polymers, suspension stabilizers, buffering agents, sweeteners, antioxidants, flavoring agents, viscosity modifying agent, surfactants, preservatives, plasticizing agent etc.
- Sustained release oral liquid suspension dosage form for once daily or twice daily administration preferably once daily administration of present invention in general comprises active ingredient in particles, granules, pellets, beads or micro particles which would be additionally mixed with appropriate additives such as viscosity modifying agents that provide suspending properties in the liquid dosage form, sweetening agents which would mask undesirable taste and feel of active ingredient, glidants which provides flow properties and also prevents caking when present in liquid composition, and also other additives such as buffering agents, lubricants, surfactants etc.
- appropriate additives such as viscosity modifying agents that provide suspending properties in the liquid dosage form, sweetening agents which would mask undesirable taste and feel of active ingredient, glidants which provides flow properties and also prevents caking when present in liquid composition, and also other additives such as buffering agents, lubricants, surfactants etc.
- sustained-release dosage forms as used in the present invention to define a release profile of an active agent over an extended period of time.
- modified-release controlled-release
- prolonged-release extended-release
- sustained-release sustained-release
- delayed release are used interchangeably herein.
- the sustained release liquid oral suspension dosage form comprises of inert pellet, active ingredient, rate controlling polymer and other pharmaceutically acceptable excipients.
- the sustained release liquid oral suspension dosage form comprises of inert pellet, high solublity active ingredient, rate controlling polymer and other pharmaceutically acceptable excipients.
- the seal coated inert pellets are loaded with active ingredient along with pharmaceutically acceptable additives, and these drug loaded pellets are further coated with rate controlling polymers to yield sustained release pellets of the active ingredient.
- the seal coated inert pellets are coated by drug coating.
- the drug coated pellets are further coated with rate controlling polymer coating.
- the sustained release pellets obtained can be further coated with drug- binder solution.
- the pellets so obtained may be coated with alkali soluble coating and/or with an acid soluble polymer coating.
- sustained release pellets which comprises:
- Drug layer surrounding the seal coated inert pellets comprises pharmaceutically active ingredient with one or more pharmaceutically acceptable excipient
- Coating layer surrounding the drug layer comprises rate controlling polymer
- sustained release pellets are suspended with viscosity modifying agent or suspending agent or thickening agent or suspension stabilizers in addition to other pharmaceutically acceptable excipients in a suspending media at a suitable pH which is maintained with or without buffer,
- sustained release liquid oral suspension does not required reconstitution before oral administration.
- sustained release pellets so obtained are further surrounded by protective coating.
- the protective coating is applied to surround a coating layer having rate controlling polymers and/or secondry drug coating or any other suitable step in process according to the present invention.
- the seal coated inert pellets are coated by drug resin complex.
- the sustained release pellets so obtained are suspended with viscosity modifying agent or suspending agent or thickening agent along with sweetener, flavoring agent, buffering agent, preservative, humectant etc in addition to other pharmaceutically acceptable excipients.
- the ready to use suspension can be prepared in aqueous or non-aqueous media at adequate pH which can be maintained with or without buffer. The preferred pH of such suspension would be in a range of 6 to 7.5.
- the dosage form can be prepared as per the therapeutic dosage requirement of particular active ingredient and to be converted in to mg/ml basis.
- “Seal coated inert pellet” are inert pellets coated with a polymer, for eg. ethylcellulose.
- the seal coating provides a film over the pellets, which improves the strength of pellets. So, during the coating/layering process the pellet does not get deformed or distorted.
- Drug-binder solution is a solution of drug and one or more binding agent.
- High solubility active ingredient is an active agent that from less than 1 part to 30 parts of water will be required to dissolve 1 part of active ingredient.
- the "pharmaceutically active ingredient” "active ingredient” or “drug substances” or “drugs” according to the present invention is selected from the group of analgesic; antiallergic; anti-anginal; antibacterial; anticonvulsant; antipsychotic; antidepressant; antidiabetic; anti-epileptic; hypolipidemic; antihyperlipidemic; hypocholesterolemic; antihypertensive; vasoconstrictor; vasodilator; anti-inflammatory; antineoplastic, antiparasitic; antiproliferative; antisecretory; antithrombotic; anti-ulcerative; antiviral; appetite suppressant; diagnostic aid; diuretic; glucocorticoid; immunizing agent; immunomodulator; immunoregulator; immunostimulant; immunosuppressant; neuroprotective; NMDA antagonist; radioactive agent; sedative; sedative-hypnotic; steroid; tranquilizer; cerebral ischemia agent; wound healing agent; xanthine
- the active ingredient which can be used in the present invention is selected from but not limited to aspirin, paracetamol, diclofenac, naproxen, oxycodone, hydrocodone, diamorphine, carbamazepine, topiramate, lamotrigine, Levetiracetam, Brivaracetam, seletracetam, primidone, clonazepam, diazepam, oxcarbazepine, zonisamide, pregabalin, ethosuximide, lorazepam, nitrazepam, midazolam, lorazepam, tolbutamide, acetohexamide, tolazamide, chlorpropamide, metoprolol, metformin, phenformin, buformin, rosiglitazone, pioglitazone,troglitazone, miglitol, acarbose, alogliptin, vildagliptin, sitagliptin,
- inert core or “inert cores” or “inert particles” or “inert pellets” herein refers to inert particles made from sugar spheres also known as non-peril seeds or other equivalents conventionally used for the preparation of dosage form like microcrystalline cellulose, dibasic calcium phosphate, mannitol or other suitable polyols or glass beads and the like.
- the “rate controlling polymer” according to the present invention can be selected from hydrophilic polymer, hydrophobic polymer or the mixture thereof.
- the rate controlling polymer can be used as a matrixing agent or as a coating agent.
- Hydrophilic polymer that can be used in the present invention can be exemplified as hydroxyethyl cellulose, hydroxypropyl cellulose, sodium alginate, carbomer, sodium carboxymethyl cellulose, xanthan gum, guar gum, locust bean gum, polyvinyl alcohol and hydroxypropyl methylcellulose.
- the preferred hydrophilic rate controlling polymer that can be used in the present invention is hydroxypropyl cellulose.
- the matrix forming polymer comprises from about 1% to about 70%, preferably from about 5% to about 50% and most preferably from 15% to 50% by weight of the coated sustained release composition.
- the hydrophilic polymer described above can also be used as a constituent of the coating layer.
- Hydrophobic polymer that can be used in the present invention are selected from the group comprising of cellulose ether such as ethyl cellulose, cellulose acetate, polyvinyl acetate, methacrylic acid esters neutral polymer, polyvinyl alcohol-maleic anhydride copolymers, hydroxypropyl methyl cellulose phthalate, EUDRAGIT ® RSPO, EUDRAGIT ® S 100, hydrogenated castor oil, waxes and the like.
- cellulose ether such as ethyl cellulose, cellulose acetate, polyvinyl acetate, methacrylic acid esters neutral polymer, polyvinyl alcohol-maleic anhydride copolymers, hydroxypropyl methyl cellulose phthalate, EUDRAGIT ® RSPO, EUDRAGIT ® S 100
- EUDRAGIT ® S 100 hydrogenated castor oil, waxes and the like.
- the commercially available dispersion of film formers namely, EUDRAGIT ® L-30D, EUDRAGIT ® NE 30D, AQUACOAT ® ECD-30, SURELEASE ® E-7, EUDRAGIT ® RS 30D, EUDRAGIT ® RL 30D, etc. may be used for the purpose of providing sustained release composition.
- the hydrophobic polymer that can be used in the present invention is ethyl cellulose, hydroxypropyl methyl cellulose phthalate, EUDRAGIT ® RSPO, EUDRAGIT ® S 100 and hydrogenated castor oil.
- Acid soluble polymer that can be used in the present invention is selected from the group of EUDRAGIT ® E 100, EUDRAGIT ® E 12, 5 and EUDRAGIT ® EPO.
- EUDRAGIT ® E 100 is used as the acid soluble polymer.
- These polymers also provide pH dependent drug release, protection of sensitive active ingredient, taste and odor masking, moisture protection etc.
- EUDRAGIT ® E 100 is used as the acid soluble polymer.
- Alkali-soluble polymers which can be used in the present invention are selected from EUDRAGIT ® S or L, EUDRAGIT ® - SI 00, EUDRAGIT ® LI 00, EUDRAGIT ® LI 00-55 EUDRAGIT ® L30D, hydroxypropyl methyl cellulose phthalate etc.
- EUDRAGIT ® LI 00-55 is used as the alkali soluble polymer.
- the polymers described above may also be used as a constituent of protective coating, which protects drug leaching.
- the protective coating may be applied as one or more layer based on characteristic of active ingredient.
- Preferred protective coating in the present invention is EUDRAGIT ® E 100 or EUDRAGIT ® RSPO or EUDRAGIT ® L100-55.
- the other pharmaceutically acceptable excipients that can be used in the present invention may be selected from filler, binder, lubricant, glidant, viscosity modifying agent, plasticizer, stabilizer, ion exchange resin, preservative, buffering agent, sweetener, flavoring agent or a mixture thereof.
- Filler which can be used in the present invention is selected from the group comprising of cellulose and cellulose derivatives like microcrystalline cellulose, calcium carbonate, calcium phosphate, dibasic calcium phosphate, tribasic calcium sulfate, calcium carboxymethylcellulose, cellulose, dextrin derivatives, dextrin, dextrose, fructose, lactitol, lactose, magnesium carbonate, magnesium oxide, maltitol, maltodextrins, maltose, sorbitol, starch, sucrose, sugar, and xylitol and other materials known to one of ordinary skill in the art or mixture thereof.
- the filler may be present in an amount from about 1% to about 50%, preferably from about 10% to about 50% by weight of the sustained release composition.
- Binder or binding agent that can be used in the present invention is selected from the group comprising of polyvinyl pyrrolidone, hydroxypropyl cellulose, hydroxypropyl methylcellulose (low viscosity grade), methyl cellulose, ethyl cellulose, starch, pregelatinized starch, modified corn starch, polyacryl amide, poly-N-vinyl amide, sodium carboxymethyl cellulose, polyethylene glycol, gelatin, polyethylene oxide, poly propylene glycol, tragacanth, alginic acid, and other materials known to one of ordinary skill in the art or mixture there of.
- the binder may be present in an amount from about 0.1% to about 20%, preferably from about 1% to about 10% by weight of the sustained release composition.
- Lubricant or glidant that can be used in the present invention is exemplified by magnesium stearate, calcium stearate, glyceryl stearate, zinc stearate, talc, polyethylene glycols, hydrogenated vegetable oil, mineral oil, stearic acid, glyceryl behenate, cornstarch, calcium silicate, magnesium silicate, colloidal silicon dioxide, silicon hydrogel, sodium stearyl fumarate, glyceryl palmitostearate, stearic acid, and other materials known to one of ordinary skill in the art or mixture thereof.
- the glidant, lubricant and anti adherent are individually present in the range from about 0.01% to about 20%w/w by weight of the sustained release composition.
- Plasticizers are used in the present formulation for providing flexibility to the sustained release polymer coating layer.
- Different kinds of plasticizers that can be used in the present formulation are selected from low molecular weight polymers, oligomers, copolymers, oils, small organic molecules, low molecular weight polyols having aliphatic hydroxyls, ester-type plasticizers, glycol ethers, polypropylene glycol, multi-block polymers, single block polymers, low molecular weight poly(ethylene glycol), citrate ester-type plasticizers, triacetin, propylene glycol and glycerin.
- plasticizers can also include ethylene glycol, 1 ,2-butylene glycol, 2,3-butylene glycol, styrene glycol, diethylene glycol, triethylene glycol, tetraethylene glycol and other poly(ethylene glycol) compounds, monopropylene glycol, monoisopropyl ether, propylene glycol monoethyl ether, ethylene glycol monoethyl ether, diethylene glycol monoethyl ether, sorbitol lactate, ethyl lactate, butyl lactate, ethyl glycolate, dibutyl sebacate, acetyltributylcitrate, triethyl citrate, acetyl triethyl citrate, tributyl citrate and allyl glycolate.
- the combination of the plasticizers can be used in the present composition.
- the composition in the present embodiment preferably comprises up to 1- 25 % of plasticizer by the total weight of the composition.
- Viscosity modifying agent or “thickening agent” or “suspending agent” are one of the most important constituent of liquid sustained release dosage form. These agents are also called as suspension stabilizers and they are intended to ensure that the individual doses removed have constant active ingredient content.
- colloidal silicates having a high aluminum and magnesium content such as bentonite, Veegum or Gel White
- colloidal silica for example Aerosil (Degussa), Cabosil (Cabot)
- organic stabilizers for example swelling agents, such as alginates, sodium alginate, calcium alginate or propylene glycol alginate, gum arabic, tragacanth, karaya gum, sterculia gum, carrageen, guar gum, xanthan gum or agar
- synthetic or semi synthetic swelling agents for example 1,2-epoxide
- polymers in particular ethylene oxide homopolymer having a degree of polymerization of about 2,000-100,000, which are known, for example, under the trade name Polyox (Union Carbide), preferably swellable cellulose ethers, for example methyl- or ethyl cellulose, hydroxy ethyl cellulose, hydroxypropyl cellulose,
- Suspension stabilizers are advantageously present in an amount which ranges up to 1- 25% by weight of the composition, preferably about 1 to about 15 % by weight.
- Ion-exchange resin that can be used in the present invention is selected from cationic and anionic ion-exchange resins.
- Ion exchange resins useful in the practice of the present invention include cationic resins such as: AMBERLITE ® IRP-64 (a porous copolymers of methacrylic acid crosslinked with divinylbenzene), and AMBERLITE ® IRP-69 (Sodium polystyrene sulfonate USP). AMBERLITE ® IRP 69 is preferred resin.
- the DOWEX ® resins, available from the Dow Chemical Company, Midland, MI are also included in the practice of the present invention. Said DOWEX ® resins are strong cationic exchangers based upon polystyrenesulphonic acid with variable crosslinking (1 -12% divinylbenzene) in a variety of particle sizes.
- AMBERLITE ® IRP 69 sodium polystyrenesulfonate
- the ion exchange resin useful in the practice of the present invention comprise from I % to 60% by weight of the pharmaceutical compositions of the present invention. More preferably, the ion exchange resins useful in the practice of the present invention comprise from about 5% to 40% by weight of the pharmaceutical compositions of the present invention.
- sustained release granules or particles prepared according to any of the above described embodiments is suspended along with other additives in an aqueous or non-aqueous solvent or solution or dispersing agent or suspending media.
- aqueous solvent or non-aqueous solvent dispersing agent or suspending media which can be used are selected from water, alcohols, oils or mixtures thereof.
- Edible oils that can be used in the present invention are selected from edible oils of animal or plant origin, such as but not limited to mono, di, and triglycerides, acetylated monoglycerides, pharmaceutically acceptable esters of aliphatic hydroxyacids, fatty acids; tocopherol and tocopherol esters, glycol esters, squalane, squalene, corn oil, limonene, crill oil, oregano oil and lipid soluble vitamins and the like.
- a common problem associated with liquid pharmaceutical dosage forms is the often disagreeable taste which may manifest itself when the drug is administered in a liquid dosage form.
- Levetiracetam is a drug with an undesirable taste. This taste may be overcome by the addition of sweeteners or flavoring agents to the formulation which mask the bitter or unpleasant taste of the drugs.
- Sweeteners are used to mask the bitter taste of the active ingredients or to impart sweetness to the liquid dosage form.
- Sweeteners that can be used in accordance with the present invention are selected from sugar such as monosaccharide or disaccharides, for example D-glucose, D-fructose, D-xylose, maltose or sucrose; polyols, such as glycerol, dulcitol, mannitol, sorbitol or xylitol, or artificial sweeteners, such as saccharine or the corresponding sodium, potassium or calcium salt, cyclamate or the corresponding sodium or calcium salt, aspartame, or acesulfame or the potassium salt thereof, furthermore Dulcin or ammonium glycyrrhizinate.
- Sweeteners are present in higher percentage in the suspension dosage form.
- Sweeteners used in the present formulation ranges from 2 to 60 % of the weight of the dosage form, preferably 5 to 50 %, more preferably
- Flavouring agents that can be used in the present invention are selected from without any limitation peppermint flavour, mint flavour, orange flavour, lemon flavour, grape flavor etc. Flavouring agents can be used in the range of 0.01 to 5% of the dosage form.
- buffering agent refers to an agent or a mixture of agents that can maintain the original acidity or basicity of a composition.
- Representative buffering agents include, but are not limited to, citric acid, sodium citrate, sodium phosphate, potassium citrate, and mixtures thereof.
- a preferred buffering agent of the present invention is a mixture of citric acid and sodium citrate. Buffering agent that can be used in the present invention ranges from 0.01 to 20%, preferably 1 to 10% by weight of the composition.
- preservative refers to an agent or mixture of agents that is used to protect a composition against antimicrobial (e.g., yeast, mold, bacteria) activity.
- Representative preservatives include, but are not limited to, sodium benzoate, benzoic acid, ethylenediaminetetraacetic acid, sorbic acid, benzethonium chloride, benzalkonium chloride, bronopol, butyl paraben, methyl paraben, ethyl paraben, propyl paraben, thiomersal, sodium propionate, chlorhexidine, chlorobutanol, chlorocresol, cresol, imidurea, phenol, phenylmercuric salts, potassium sorbate, propylene glycol, and mixtures thereof.
- a preferred preservative of the present invention is the combination of methyl paraben and propyl paraben. Preservatives that can be used in the present invention ranges from 0.01% to 5%.
- a humectant is a hygroscopic substance. It is often a molecule with several hydrophilic groups, most often hydroxyl groups, but amines and carboxyl groups, sometimes esterified, can be encountered as well. Humectants maintain the water content of the liquid dosage form. Examples of humectants include glycerin, propylene glycol and glyceryl triacetate. Others can be polyols like sorbitol, xylitol and maltitol, polymeric polyols like polydextrose, or natural extracts like quillaia, lactic acid or urea. Preferably maltitol and glycerin is used as humectant in the present invention. The amount of preservatives that can be used in the present invention ranges from 0.01% to 5%.
- the sustained release oral suspension can be prepared by the following process;
- Suitable fillers are coated with a binder solution to obtain seal coated inert pellets.
- step b) Seal coated inert pellets obtained from the above step are further coated with a drug -binder solution or drug-binder resin complex to form drug layering, c) Drug layered pellets so obtained from step b) are further coated with a rate controlling polymer with one or more pharmaceutically acceptable excipient to form coated layer.
- step c) the pellets so obtained in step c) are further coated by drug binder solution or drug resin complex,
- pellets so obtained either from step c) or d) are further coated with protective coating using suitable polymer.
- Sustained release pellets so obtained are suspended with viscosity modifying agent or suspending agent or thickening agent or suspension stabilizers along with sweeteners, flavoring agent, buffering agent, preservative, humectants in addition to other pharmaceutically acceptable excipients in aqueous or non aqueous media at a pH which can be maintained with or without buffer.
- the sustained release oral suspension can be prepared by the following process;
- Suitable fillers are coated with a binder solution to obtain seal coated inert pellets.
- Seal coated inert pellets obtained from the above step are further coated with a drug - binder solution or drug-binder resin complex to form drug layering.
- step b) Drug layered pellets so obtained from step b) are further coated with a rate controlling polymer with one or more pharmaceutically acceptable excipient to form coated layer.
- step c) the pellets so obtained in step c) are further coated by drug binder solution or drug resin complex.
- step e) The pellets so obtained from step c) or d) are further coated with protective coating.
- the pellets so obtained either from step e) are optionally coated using acid soluble or/and alkali soluble polymer.
- Sustained release pellets so obtained are suspended with viscosity modifying agent or suspending agent or thickening agent or suspension stabilizers along with sweeteners, flavoring agent, buffering agent, preservative, humectants in addition to other pharmaceutically acceptable excipients in aqueous OR non aqueous media at a pH which can be maintained with or without buffer.
- Seal coating Sifted the sugar pellets through the 60/80 mesh. Ethylcellulose (EC) and talc are dispersed in a blend of Methanol/Dichloromethane (DCM). This dispersion was coated on spheres using FBC bottom spray to get seal coated pellets.
- EC Ethylcellulose
- DCM Methanol/Dichloromethane
- Extended release layering Dispersed Diethyl phthatate (DEP), Triethyl citrate (TEC), HPMC 6 cps and Ethyl cellulose (EC) 45 cps in methanol with stirring to get uniform dispersion. Methylene chloride was added to this dispersion to solubilize all dispersed material by stirring. Talc and Ferric oxide yellow are mixed with stirring. Drug loaded pellets were coated with this suspension using FBC bottom spray to get extended release drug pellets.
- Suspension vehicle preparation Hydrogenated castor oil, aerosil with corn oil was milled in colloid mill. Continue the milling and added propyl gallate, methyl paraben, propyl paraben and sucralose. Finally the color (Lake of Panceau) and flavors (strawberry) are milled. Filter this milled suspension through # 40 sieve.
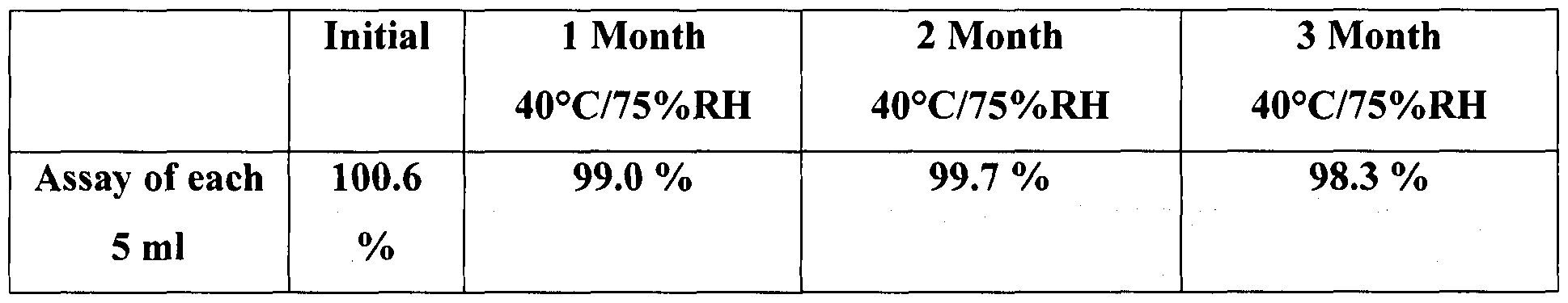
- the dissolution data (Table 4) for levetiracetam sustained release suspension at different time periods shows that there is no significant change in dissolution of 40°C/75%RH, 1 , 2, 3 Months as compared to the initial data.
- Extended release layering Dispersed Diethyl phthatate (DEP), Triethyl citrate (TEC), HPMC 6 cps and Ethyl cellulose (EC) 45 cps in methanol with stirring to get uniform dispersion. Methylene chloride was added to this dispersion to solubilize all dispersed material by stirring. Talc and Ferric oxide yellow are mixed with stirring. Drug loaded pellets were coated with this suspension using FBC bottom spray to get extended release drug pellets.
- Suspension vehicle preparation Hydrogenated castor oil, aerosol,xanthan gum with purified water was milled in colloid mill. Continue the milling and added propyl gallate, methyl paraben, propyl paraben and sucrose/asparmate. Finally the color (Lake of Panceau) and flavors (strawberry) are milled. Filter this milled suspension through # 40 sieve.
- Microcrystalline cellulose pellets (#70- 140) 714.285
- Microcrystalline cellulose pellets ( # 60- 100) 750.00
- Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
- Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
- Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
- Sustained release pellets obtained as above are again coated with the dispersion obtained by adding Eudragit E 100, talc, diethyl phthalate in a mixture of isopropyl alcohol and acetone.
- Example 4 Sustained release suspension of Levetiracetam by hydrogenated castor oil.
- Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
- Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
- Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
- Ethyl cellulose pellets are further coated with hydrogenated castor oil.
- pellets obtained from the above step are coated with alkaline soluble polymer Eudragit E 100.
- pellets thus obtained are suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
- Example -5 Sustained release liquid dosage form having alkali soluble coating and acid soluble coating.
- Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
- Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
- Drug loaded pellets are coated with dispersion of Eudragit RSPO obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
- Eudragit RSPO pellets are further coated with an alkaline soluble coating comprising of Eudragit L 100-55.
- pellets obtained from the above step are coated with acid soluble polymer Eudragit E 100.
- pellets thus obtained are suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
- Example -6 Sustained release liquid suspension having dual drug release.
- Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
- Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
- Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
- Drug-binder solution obtained by dissolving levetiracetam, HPMC, talc in purified water is sprayed upon the sustained release pellets obtained above.
- pellets obtained from the above step are coated with alkaline soluble polymer Eudragit L 100-55.
- Example -7 Sustained release suspension prepared by ion-exchange resin complexation.
- Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
- Drug-resin complex solution is prepared by dissolving levetiracetam, ion-exchange resin, talc and HPMC in purified water. The drug-resin complex binder dispersion was sprayed upon the seal coated pellets.
- Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
- the pellets thus obtained can be suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
Abstract
A ready to use stable, sustained release oral liquid suspension dosage form of pharmaceutical active ingredients, which is easy to administer and particularly beneficial for the pediatric and geriatric patients. The suspension dosage form comprises sustained release pellets comprising inert pellets, surrounded by seal coating, drug layer comprising pharmaceutically active ingredient with one or more pharmaceutically acceptable excipients surrounding said seal coated inert pellets, and coating layer comprising rate controlling polymer surrounding said drug layer, such that the sustained release pellets are suspended with suitable suspending agent, in addition to other pharmaceutically acceptable excipients in a suspending media at a suitable pH. A process for preparation of the suspension dosage form is also provided.
Description
SUSTAINED RELEASE ORAL LIQUID SUSPENSION DOSAGE FORM
FIELD OF THE INVENTION
The present invention relates to a stable, sustained release oral liquid suspension dosage form of pharmaceutical active ingredients, which is easy to administer and particularly beneficial for the pediatric and geriatric patients. This invention particularly relates to a sustained release oral liquid suspension dosage form which is a ready-to-use and does not require reconstitution before oral administration. Present invention also relates to a process for preparing said dosage form.
BACKGROUND
Oral route is the most convenient and preferred route for the drug administration and there are various conventional dosage forms available for the oral administration of the drug such as tablets, capsules, solutions, emulsions or suspensions. However, for the pediatric and geriatric patients solid oral dosage formulations especially large tablets or capsules are very difficult to swallow, hence for such patients liquid formulations are most preferred dosage forms and patients may be more inclined to comply with their medication instructions if the dosages are easier to ingest.
However, the problem with the use of conventional oral dosage forms like tablets, capsules and solution is the fluctuation in the blood concentrations within each interval between dosing. Moreover, certain drugs, especially those exhibiting a short half-life necessitate frequent dosing, are too rapidly absorbed and sometimes result in peak serum levels that are excessively high. Such fluctuating serum drug levels often result in the development of adverse symptoms. Thus the pharmaceutical scientists have developed formulations to prolong the residence time of drug molecules in the body by controlling the release rate of the drug from the formulation to enhance convenience of the regimen, improve patient compliance and reduce frequency of side effects due to fluctuations in blood concentration. Further, by controlling the release of the active substance it is possible to influence the extent of absorption and the duration of action.
For many drugs sustained release solid dosage forms are available in the market such as extended release tablets of Levetiracetam (Keppra®), Metformin (Fortamet®), Metoprolol
(Toprol-XL®), Desvenlafaxine (Pristiq®), and Venlafaxine (Effexor XR®). However such controlled release solid formulations requires large amounts of excipients which can retard the drug release in the formulation such as polymers, waxes as matrixing agents and increases the size of the final formulation which reduces patient compliance especially for the pediatric and geriatric patients. Therefore an alternative approach to administer sustained release dosage form will be an oral liquid dosage form. Among the liquid dosage form, the suspension formulation against the solution can provide sustained drug release by controlling the drug release. Such formulations are particularly suitable for pediatric, geriatric patients and for those who can not swallow the tablet dosage form. However, there are different challenges in preparation of such dosage form and thus there are very few sustained release suspension dosage form products available commercially in the market. The major challenge with suspension of sustained release dosage form would be to maintain the stability of suspension throughout the shelf life because in case of failure, irregular blood concentration may be achieved and can be sometimes sub therapeutic or toxic.
The inventors of the present inventions have discovered a stable, taste masked oral sustained release dosage form.
Various approaches are disclosed for oral sustained release dosage form in the literature. US5296236 discloses liquid- controlled release suspension of the drug containing micro granules coated with series of successive polymer having alternate hydrophobic and hydrophilic coatings. US 4999189 disclose sustained release oral suspensions wherein drug- resin particle coated with a first inner coating of water-insoluble wax and a second outer coating of a pharmaceutically acceptable water-insoluble polymer. WO2006093838 discloses method of preparing controlled release suspension of drug microbeads in thixotropic solution. US5156842 discloses oral sustained release suspension of the antibiotics, the antibiotic of said microparticles being coated with, distributed through or adsorbed onto the non-toxic polymer and finally such coated microparticles are suspended in of non-aqueous vehicles. EP0717992 discloses aqueous pharmaceutical suspensions containing a high amount of acetaminophen embedded in a polymer to impart controlled release properties. US20091 1 1872 discloses stabilized pharmaceutical suspension of carisbamate using
hypromellose. US20030108575 mentions oral suspension formulation for the drug of low water solubility using wetting agent, thixotropic thickening agent and suspending agent. WO2003053403 discloses use of water-soluble or swellable nonsurfactant polymer along with the suspending agents for the poorly water soluble drug for the suspension formulation. US7300670 discloses an aqueous suspension of the particulate drug containing suspending agent in a liquid phase. WO2008122993 discloses drug containing microparticles with first pH independent polymer coating and second pH dependent polymer coating layer. US 6156340 disclose drug liquid sprayed on to inert core and such drug coated particles were further coated with two layers of the polymer having increasing permeability for the water.
SUMMARY OF THE INVENTION
The present invention relates to a stable, sustained release oral liquid suspension dosage form of pharmaceutical active ingredients.
The present invention is to provide a sustained release oral liquid suspension dosage form of pharmaceutically active ingredients, which is ready to use and does not require reconstitution before oral administration.
An another aspect of the present invention is to provide a- sustained release oral liquid suspension dosage form of active ingredients which can be administered as once-daily or twice-daily.
Another aspect of the present invention is to provide a sustained release oral liquid suspension dosage form of high solubility active ingredients, which can be administered as once-daily or twice-daily.
Another aspect of the present invention is to provide a sustained release oral liquid suspension dosage form of active ingredients, which is stable during storage period and does not leach out in suspending media during storage period.
Further aspect of the present invention is to provide a sustained release oral liquid suspension dosage form of active ingredients, which is particularly convenient for the pediatric and geriatric patient population.
Another aspect of the present invention is to provide a process for the preparation of sustained release oral liquid suspension dosage form of active ingredients.
Another aspect of the present invention is to provide a sustained release liquid suspension dosage form of active ingredients which comprises of the pharmaceutically acceptable additives like that of fillers, binders, lubricants, glidants, rate controlling polymers, suspension stabilizers, buffering agents, sweeteners, antioxidants, flavoring agents, viscosity modifying agent, surfactants, preservatives, plasticizing agent etc.
DETAIL DESCRIPTION OF THE INVENTION
Sustained release oral liquid suspension dosage form for once daily or twice daily administration, preferably once daily administration of present invention in general comprises active ingredient in particles, granules, pellets, beads or micro particles which would be additionally mixed with appropriate additives such as viscosity modifying agents that provide suspending properties in the liquid dosage form, sweetening agents which would mask undesirable taste and feel of active ingredient, glidants which provides flow properties and also prevents caking when present in liquid composition, and also other additives such as buffering agents, lubricants, surfactants etc.
"Sustained-release dosage forms" as used in the present invention to define a release profile of an active agent over an extended period of time. The terms "modified-release", controlled-release", "prolonged-release", "extended-release", "sustained-release" and "delayed release" are used interchangeably herein.
In an embodiment of the present invention the sustained release liquid oral suspension dosage form comprises of inert pellet, active ingredient, rate controlling polymer and other pharmaceutically acceptable excipients.
In an embodiment of the present invention the sustained release liquid oral suspension dosage form comprises of inert pellet, high solublity active ingredient, rate controlling polymer and other pharmaceutically acceptable excipients.
In further embodiments of the present invention, the seal coated inert pellets are loaded with active ingredient along with pharmaceutically acceptable additives, and these drug loaded pellets are further coated with rate controlling polymers to yield sustained release pellets of the active ingredient.
In another embodiment of the present invention the seal coated inert pellets are coated by drug coating. The drug coated pellets are further coated with rate controlling polymer coating. Optionally the sustained release pellets obtained can be further coated with drug- binder solution. The pellets so obtained may be coated with alkali soluble coating and/or with an acid soluble polymer coating.
In a preferred aspect the present invention provides a ready to use, stable sustained release liquid oral suspension dosage form comprises: sustained release pellets, which comprises:
a) Inert pellets, surrounded by seal coating,
b) Drug layer surrounding the seal coated inert pellets comprises pharmaceutically active ingredient with one or more pharmaceutically acceptable excipient,
c) Coating layer surrounding the drug layer comprises rate controlling polymer,
Wherein the sustained release pellets are suspended with viscosity modifying agent or suspending agent or thickening agent or suspension stabilizers in addition to other pharmaceutically acceptable excipients in a suspending media at a suitable pH which is maintained with or without buffer,
Wherein the sustained release liquid oral suspension does not required reconstitution before oral administration.
In another preferred aspect, the sustained release pellets so obtained are further surrounded by protective coating.
In another preferred aspect the protective coating is applied to surround a coating layer having rate controlling polymers and/or secondry drug coating or any other suitable step in process according to the present invention.
In another preferred embodiment of the present invention the seal coated inert pellets are coated by drug resin complex.
The sustained release pellets so obtained are suspended with viscosity modifying agent or suspending agent or thickening agent along with sweetener, flavoring agent, buffering agent, preservative, humectant etc in addition to other pharmaceutically acceptable excipients. The ready to use suspension can be prepared in aqueous or non-aqueous media at
adequate pH which can be maintained with or without buffer. The preferred pH of such suspension would be in a range of 6 to 7.5.
The dosage form can be prepared as per the therapeutic dosage requirement of particular active ingredient and to be converted in to mg/ml basis.
"Seal coated inert pellet" according to the present invention are inert pellets coated with a polymer, for eg. ethylcellulose. The seal coating provides a film over the pellets, which improves the strength of pellets. So, during the coating/layering process the pellet does not get deformed or distorted.
"Drug-binder solution" according to the present invention is a solution of drug and one or more binding agent.
"High solubility active ingredient" according to the present invention is an active agent that from less than 1 part to 30 parts of water will be required to dissolve 1 part of active ingredient.
The "pharmaceutically active ingredient" "active ingredient" or "drug substances" or "drugs" according to the present invention is selected from the group of analgesic; antiallergic; anti-anginal; antibacterial; anticonvulsant; antipsychotic; antidepressant; antidiabetic; anti-epileptic; hypolipidemic; antihyperlipidemic; hypocholesterolemic; antihypertensive; vasoconstrictor; vasodilator; anti-inflammatory; antineoplastic, antiparasitic; antiproliferative; antisecretory; antithrombotic; anti-ulcerative; antiviral; appetite suppressant; diagnostic aid; diuretic; glucocorticoid; immunizing agent; immunomodulator; immunoregulator; immunostimulant; immunosuppressant; neuroprotective; NMDA antagonist; radioactive agent; sedative; sedative-hypnotic; steroid; tranquilizer; cerebral ischemia agent; wound healing agent; xanthine oxidase inhibitor and the like.
The active ingredient, which can be used in the present invention is selected from but not limited to aspirin, paracetamol, diclofenac, naproxen, oxycodone, hydrocodone, diamorphine, carbamazepine, topiramate, lamotrigine, Levetiracetam, Brivaracetam, seletracetam, primidone, clonazepam, diazepam, oxcarbazepine, zonisamide, pregabalin, ethosuximide, lorazepam, nitrazepam, midazolam, lorazepam, tolbutamide, acetohexamide,
tolazamide, chlorpropamide, metoprolol, metformin, phenformin, buformin, rosiglitazone, pioglitazone,troglitazone, miglitol, acarbose, alogliptin, vildagliptin, sitagliptin, saxagliptin, glipizide, glimeperide, glyburide, repaglinide, nateglinide, Chlorpromazine, Fluphenazine, Perphenazine, Prochlorperazine, Thioridazine, Trifluoperazine, Mesoridazine, Periciazine, Promazine, Triflupromazine, Levomepromazine, Promethazine, Pimozide chlorprothixene, clopenthixol, flupenthixol, thithixene, Zuclopenthixol, Clozapine, Olanzapine, Risperidone, Paliperidone, Quetiqpine, Ziprasidone, Amisulpride, Asenapine, Iloperidone, Zotepine, Sertindole, Lurasidone, Aripiprazole, alprazolam, Zolpidem, bupropion, duloxetine, milnacipran, tolterodine, diltiazem, Oxybutinin, venlafaxine, desvenlafaxine, tramadol, tapentadol or there pharmaceutical acceptable salts and a combination thereof.
The term "inert core" or "inert cores" or "inert particles" or "inert pellets" herein refers to inert particles made from sugar spheres also known as non-peril seeds or other equivalents conventionally used for the preparation of dosage form like microcrystalline cellulose, dibasic calcium phosphate, mannitol or other suitable polyols or glass beads and the like.
The use of the terms "a" and "an" and "the" and similar referents in the context of describing the invention (especially in the context of the following claims) are to be construed to cover both the singular and the plural, unless otherwise indicated herein or clearly contradicted by context.
The "rate controlling polymer" according to the present invention can be selected from hydrophilic polymer, hydrophobic polymer or the mixture thereof. The rate controlling polymer can be used as a matrixing agent or as a coating agent. Hydrophilic polymer that can be used in the present invention can be exemplified as hydroxyethyl cellulose, hydroxypropyl cellulose, sodium alginate, carbomer, sodium carboxymethyl cellulose, xanthan gum, guar gum, locust bean gum, polyvinyl alcohol and hydroxypropyl methylcellulose. The preferred hydrophilic rate controlling polymer that can be used in the present invention is hydroxypropyl cellulose. The matrix forming polymer comprises from about 1% to about 70%, preferably from about 5% to about 50% and most preferably from 15% to 50% by
weight of the coated sustained release composition. The hydrophilic polymer described above can also be used as a constituent of the coating layer.
Hydrophobic polymer that can be used in the present invention are selected from the group comprising of cellulose ether such as ethyl cellulose, cellulose acetate, polyvinyl acetate, methacrylic acid esters neutral polymer, polyvinyl alcohol-maleic anhydride copolymers, hydroxypropyl methyl cellulose phthalate, EUDRAGIT® RSPO, EUDRAGIT® S 100, hydrogenated castor oil, waxes and the like. Even the commercially available dispersion of film formers namely, EUDRAGIT® L-30D, EUDRAGIT® NE 30D, AQUACOAT® ECD-30, SURELEASE® E-7, EUDRAGIT® RS 30D, EUDRAGIT® RL 30D, etc. may be used for the purpose of providing sustained release composition. Preferably the hydrophobic polymer that can be used in the present invention is ethyl cellulose, hydroxypropyl methyl cellulose phthalate, EUDRAGIT® RSPO, EUDRAGIT® S 100 and hydrogenated castor oil.
Acid soluble polymer that can be used in the present invention is selected from the group of EUDRAGIT® E 100, EUDRAGIT® E 12, 5 and EUDRAGIT® EPO. Preferably EUDRAGIT® E 100 is used as the acid soluble polymer. These polymers also provide pH dependent drug release, protection of sensitive active ingredient, taste and odor masking, moisture protection etc. Preferably EUDRAGIT® E 100 is used as the acid soluble polymer.
Alkali-soluble polymers which can be used in the present invention are selected from EUDRAGIT® S or L, EUDRAGIT®- SI 00, EUDRAGIT® LI 00, EUDRAGIT® LI 00-55 EUDRAGIT® L30D, hydroxypropyl methyl cellulose phthalate etc. Preferably EUDRAGIT® LI 00-55 is used as the alkali soluble polymer.
The polymers described above may also be used as a constituent of protective coating, which protects drug leaching. The protective coating may be applied as one or more layer based on characteristic of active ingredient. Preferred protective coating in the present invention is EUDRAGIT® E 100 or EUDRAGIT® RSPO or EUDRAGIT® L100-55.
The other pharmaceutically acceptable excipients that can be used in the present invention may be selected from filler, binder, lubricant, glidant, viscosity modifying agent,
plasticizer, stabilizer, ion exchange resin, preservative, buffering agent, sweetener, flavoring agent or a mixture thereof.
Filler which can be used in the present invention is selected from the group comprising of cellulose and cellulose derivatives like microcrystalline cellulose, calcium carbonate, calcium phosphate, dibasic calcium phosphate, tribasic calcium sulfate, calcium carboxymethylcellulose, cellulose, dextrin derivatives, dextrin, dextrose, fructose, lactitol, lactose, magnesium carbonate, magnesium oxide, maltitol, maltodextrins, maltose, sorbitol, starch, sucrose, sugar, and xylitol and other materials known to one of ordinary skill in the art or mixture thereof. The filler may be present in an amount from about 1% to about 50%, preferably from about 10% to about 50% by weight of the sustained release composition.
Binder or binding agent that can be used in the present invention is selected from the group comprising of polyvinyl pyrrolidone, hydroxypropyl cellulose, hydroxypropyl methylcellulose (low viscosity grade), methyl cellulose, ethyl cellulose, starch, pregelatinized starch, modified corn starch, polyacryl amide, poly-N-vinyl amide, sodium carboxymethyl cellulose, polyethylene glycol, gelatin, polyethylene oxide, poly propylene glycol, tragacanth, alginic acid, and other materials known to one of ordinary skill in the art or mixture there of. The binder may be present in an amount from about 0.1% to about 20%, preferably from about 1% to about 10% by weight of the sustained release composition.
Lubricant or glidant that can be used in the present invention is exemplified by magnesium stearate, calcium stearate, glyceryl stearate, zinc stearate, talc, polyethylene glycols, hydrogenated vegetable oil, mineral oil, stearic acid, glyceryl behenate, cornstarch, calcium silicate, magnesium silicate, colloidal silicon dioxide, silicon hydrogel, sodium stearyl fumarate, glyceryl palmitostearate, stearic acid, and other materials known to one of ordinary skill in the art or mixture thereof. The glidant, lubricant and anti adherent are individually present in the range from about 0.01% to about 20%w/w by weight of the sustained release composition.
Plasticizers are used in the present formulation for providing flexibility to the sustained release polymer coating layer. Different kinds of plasticizers that can be used in the present formulation are selected from low molecular weight polymers, oligomers,
copolymers, oils, small organic molecules, low molecular weight polyols having aliphatic hydroxyls, ester-type plasticizers, glycol ethers, polypropylene glycol, multi-block polymers, single block polymers, low molecular weight poly(ethylene glycol), citrate ester-type plasticizers, triacetin, propylene glycol and glycerin. Such plasticizers can also include ethylene glycol, 1 ,2-butylene glycol, 2,3-butylene glycol, styrene glycol, diethylene glycol, triethylene glycol, tetraethylene glycol and other poly(ethylene glycol) compounds, monopropylene glycol, monoisopropyl ether, propylene glycol monoethyl ether, ethylene glycol monoethyl ether, diethylene glycol monoethyl ether, sorbitol lactate, ethyl lactate, butyl lactate, ethyl glycolate, dibutyl sebacate, acetyltributylcitrate, triethyl citrate, acetyl triethyl citrate, tributyl citrate and allyl glycolate. Also the combination of the plasticizers can be used in the present composition. The composition in the present embodiment preferably comprises up to 1- 25 % of plasticizer by the total weight of the composition.
"Viscosity modifying agent" or "thickening agent" or "suspending agent" are one of the most important constituent of liquid sustained release dosage form. These agents are also called as suspension stabilizers and they are intended to ensure that the individual doses removed have constant active ingredient content. A large variety of agents can be used for the above purpose like colloidal silicates having a high aluminum and magnesium content, such as bentonite, Veegum or Gel White; colloidal silica, for example Aerosil (Degussa), Cabosil (Cabot); organic stabilizers, for example swelling agents, such as alginates, sodium alginate, calcium alginate or propylene glycol alginate, gum arabic, tragacanth, karaya gum, sterculia gum, carrageen, guar gum, xanthan gum or agar; synthetic or semi synthetic swelling agents, for example 1,2-epoxide; polymers, in particular ethylene oxide homopolymer having a degree of polymerization of about 2,000-100,000, which are known, for example, under the trade name Polyox (Union Carbide), preferably swellable cellulose ethers, for example methyl- or ethyl cellulose, hydroxy ethyl cellulose, hydroxypropyl cellulose, hydroxypropylmethyl cellulose, methyl- or ethylhydroxyethyl cellulose, carboxymethyl cellulose or an alkali metal salt thereof, or microcrystalline cellulose, or water-soluble polyvinyl compounds, such as polyvinyl acetate, polyvinyl alcohol or
polyvinylpyrrolidone or combination thereof. Preferably xanthan gum is used as the suspension stabilizers.
Suspension stabilizers are advantageously present in an amount which ranges up to 1- 25% by weight of the composition, preferably about 1 to about 15 % by weight.
Ion-exchange resin that can be used in the present invention is selected from cationic and anionic ion-exchange resins. Ion exchange resins useful in the practice of the present invention include cationic resins such as: AMBERLITE® IRP-64 (a porous copolymers of methacrylic acid crosslinked with divinylbenzene), and AMBERLITE® IRP-69 (Sodium polystyrene sulfonate USP). AMBERLITE® IRP 69 is preferred resin. The DOWEX® resins, available from the Dow Chemical Company, Midland, MI are also included in the practice of the present invention. Said DOWEX® resins are strong cationic exchangers based upon polystyrenesulphonic acid with variable crosslinking (1 -12% divinylbenzene) in a variety of particle sizes.
Further, said AMBERLITE® IRP 69 (sodium polystyrenesulfonate) is available commercially as a sodium salt. However, it is within the scope of the present invention to convert the sodium salt to other salt forms including, but not limited to, K and Li. The ion exchange resin useful in the practice of the present invention comprise from I % to 60% by weight of the pharmaceutical compositions of the present invention. More preferably, the ion exchange resins useful in the practice of the present invention comprise from about 5% to 40% by weight of the pharmaceutical compositions of the present invention.
In an embodiment of the present invention the sustained release granules or particles prepared according to any of the above described embodiments is suspended along with other additives in an aqueous or non-aqueous solvent or solution or dispersing agent or suspending media.
According to the present invention aqueous solvent or non-aqueous solvent dispersing agent or suspending media which can be used are selected from water, alcohols, oils or mixtures thereof. Edible oils that can be used in the present invention are selected from edible oils of animal or plant origin, such as but not limited to mono, di, and triglycerides, acetylated monoglycerides, pharmaceutically acceptable esters of aliphatic hydroxyacids,
fatty acids; tocopherol and tocopherol esters, glycol esters, squalane, squalene, corn oil, limonene, crill oil, oregano oil and lipid soluble vitamins and the like.
A common problem associated with liquid pharmaceutical dosage forms is the often disagreeable taste which may manifest itself when the drug is administered in a liquid dosage form. For example, Levetiracetam is a drug with an undesirable taste. This taste may be overcome by the addition of sweeteners or flavoring agents to the formulation which mask the bitter or unpleasant taste of the drugs.
Sweeteners are used to mask the bitter taste of the active ingredients or to impart sweetness to the liquid dosage form. Sweeteners that can be used in accordance with the present invention are selected from sugar such as monosaccharide or disaccharides, for example D-glucose, D-fructose, D-xylose, maltose or sucrose; polyols, such as glycerol, dulcitol, mannitol, sorbitol or xylitol, or artificial sweeteners, such as saccharine or the corresponding sodium, potassium or calcium salt, cyclamate or the corresponding sodium or calcium salt, aspartame, or acesulfame or the potassium salt thereof, furthermore Dulcin or ammonium glycyrrhizinate. Generally sweeteners are present in higher percentage in the suspension dosage form. Sweeteners used in the present formulation ranges from 2 to 60 % of the weight of the dosage form, preferably 5 to 50 %, more preferably 5-30% weight of the composition.
Flavouring agents that can be used in the present invention are selected from without any limitation peppermint flavour, mint flavour, orange flavour, lemon flavour, grape flavor etc. Flavouring agents can be used in the range of 0.01 to 5% of the dosage form.
The term "buffering agent," as used herein, refers to an agent or a mixture of agents that can maintain the original acidity or basicity of a composition. Representative buffering agents include, but are not limited to, citric acid, sodium citrate, sodium phosphate, potassium citrate, and mixtures thereof. A preferred buffering agent of the present invention is a mixture of citric acid and sodium citrate. Buffering agent that can be used in the present invention ranges from 0.01 to 20%, preferably 1 to 10% by weight of the composition.
The term "preservative," as used herein, refers to an agent or mixture of agents that is used to protect a composition against antimicrobial (e.g., yeast, mold, bacteria) activity.
Representative preservatives include, but are not limited to, sodium benzoate, benzoic acid, ethylenediaminetetraacetic acid, sorbic acid, benzethonium chloride, benzalkonium chloride, bronopol, butyl paraben, methyl paraben, ethyl paraben, propyl paraben, thiomersal, sodium propionate, chlorhexidine, chlorobutanol, chlorocresol, cresol, imidurea, phenol, phenylmercuric salts, potassium sorbate, propylene glycol, and mixtures thereof. A preferred preservative of the present invention is the combination of methyl paraben and propyl paraben. Preservatives that can be used in the present invention ranges from 0.01% to 5%.
A humectant is a hygroscopic substance. It is often a molecule with several hydrophilic groups, most often hydroxyl groups, but amines and carboxyl groups, sometimes esterified, can be encountered as well. Humectants maintain the water content of the liquid dosage form. Examples of humectants include glycerin, propylene glycol and glyceryl triacetate. Others can be polyols like sorbitol, xylitol and maltitol, polymeric polyols like polydextrose, or natural extracts like quillaia, lactic acid or urea. Preferably maltitol and glycerin is used as humectant in the present invention. The amount of preservatives that can be used in the present invention ranges from 0.01% to 5%.
In an another embodiment of the present invention, the sustained release oral suspension can be prepared by the following process;
a) Suitable fillers are coated with a binder solution to obtain seal coated inert pellets.
b) Seal coated inert pellets obtained from the above step are further coated with a drug -binder solution or drug-binder resin complex to form drug layering, c) Drug layered pellets so obtained from step b) are further coated with a rate controlling polymer with one or more pharmaceutically acceptable excipient to form coated layer.
d) Optionally, the pellets so obtained in step c) are further coated by drug binder solution or drug resin complex,
e) The pellets so obtained either from step c) or d) are further coated with protective coating using suitable polymer.
f) Sustained release pellets so obtained are suspended with viscosity modifying agent or suspending agent or thickening agent or suspension stabilizers along with sweeteners, flavoring agent, buffering agent, preservative, humectants in addition to other pharmaceutically acceptable excipients in aqueous or non aqueous media at a pH which can be maintained with or without buffer.
In one of the preferred embodiment of the present invention, the sustained release oral suspension can be prepared by the following process;
a) Suitable fillers are coated with a binder solution to obtain seal coated inert pellets. b) Seal coated inert pellets obtained from the above step are further coated with a drug - binder solution or drug-binder resin complex to form drug layering.
c) Drug layered pellets so obtained from step b) are further coated with a rate controlling polymer with one or more pharmaceutically acceptable excipient to form coated layer.
d) Optionally, the pellets so obtained in step c) are further coated by drug binder solution or drug resin complex.
e) The pellets so obtained from step c) or d) are further coated with protective coating. f) The pellets so obtained either from step e) are optionally coated using acid soluble or/and alkali soluble polymer.
g) Sustained release pellets so obtained are suspended with viscosity modifying agent or suspending agent or thickening agent or suspension stabilizers along with sweeteners, flavoring agent, buffering agent, preservative, humectants in addition to other pharmaceutically acceptable excipients in aqueous OR non aqueous media at a pH which can be maintained with or without buffer.
The invention will be further illustrated by the following Examples, however, without restricting its scope to these embodiments.
EXAMPLES:
Example 1
Levetiracetam sustained release suspension for 100 mg/ml
Ingredients Qty in mg
Seal Coating
Sugar Spheres (#60-80) 45.45
Ethyl Cellulose 10 cps 3.64
Purified Talc 0.91
Methyl Alcohol q.s.
DCM q.s.
Levetiracetam Drug Coating
Seal coated pellets 50
Levetiracetam 100
HPMC 6 cps 18.8
Talc 6.2
Methanol q.s.
Extended release coating
Drug coated pellets 175.00
Ethyl Cellulose 45 cps 45.29
HPMC 2910 6 cps 4.53
DEP 4.53
TEC 2.26
Talc 4.53
Ferric oxide yellow 0.1 1
Methanol q.s.
DCM q.s.
Protective Coating
EC coated pellets 236.25
Eudragit RSPO 47.25
DEP 4.725
Talc 18.9
Acetone q.s
Isopropyl alcohol q.s
Preparation of Corn Oil suspension
Eudragit RSPO coated pellets 307.13
Corn Oil 523.35
Methyl Paraben 0.54
Propyl Paraben 0.54
Hydrogenated Castor Oil 1.08
Aerosil 2.74
Sucralose 2.70
Propyl gallate 1.08
Lake of Panceau 0.27
Strawberry 2.70 .
Suspension with pellets 842.12
Table 1 : Composition of Levetiracetam sustained release suspension 100 mg / ml Procedure:
1 Seal coating: Sifted the sugar pellets through the 60/80 mesh. Ethylcellulose (EC) and talc are dispersed in a blend of Methanol/Dichloromethane (DCM). This dispersion was coated on spheres using FBC bottom spray to get seal coated pellets.
2 Drug layering: Dissolved HPMC 6 cps in methanol then added Levetiracetam and Talc to this dispersion, stirred the dispersion to get homogeneous suspension. Seal coated pellets were coated with this suspension using FBC bottom spray to get drug loaded pellets.
3 Extended release layering: Dispersed Diethyl phthatate (DEP), Triethyl citrate (TEC), HPMC 6 cps and Ethyl cellulose (EC) 45 cps in methanol with stirring to get uniform
dispersion. Methylene chloride was added to this dispersion to solubilize all dispersed material by stirring. Talc and Ferric oxide yellow are mixed with stirring. Drug loaded pellets were coated with this suspension using FBC bottom spray to get extended release drug pellets.
4 Protective layering: Eudragit RSPO and DEP were dissolved in a blend of Acetone/ Isopropyl alcohol with stirring. Finally talc was dispersd in this solution with stirring. Extended release drug pellets were coated with this solution using FBP bottom spray.
5 Suspension vehicle preparation: Hydrogenated castor oil, aerosil with corn oil was milled in colloid mill. Continue the milling and added propyl gallate, methyl paraben, propyl paraben and sucralose. Finally the color (Lake of Panceau) and flavors (strawberry) are milled. Filter this milled suspension through # 40 sieve.
6. Preparation of Suspension: Mixed the drug pellets of step 4 in suspension vehicle of step 5 with stirring. The dissolution data (Table 2) for levetiracetam suspension at the initial shows that, the suspension provided a sustained release up to 12 hrs as compared with Keppra®.
Procedure: Weighed accurately about eq. to 500 mg of Levetiracetam in 100 ml beaker from the bottle (the bottle should be well shaken). Washed this suspension with 20ml n-Hexane for 3-4 times and discarded n-hexane. Allowed to stand the pellets for drying at room temperature for 1 hr. Transfer these pellets in dissolution vessel without loosing any pellets.
Time (Hrs) Levetiracetam Oil based suspension Keppra (500 mg)
Dissolution (% Release)
1 16 31
2 47 47
3 62 59
4 74 69
6 86 83
8 90 91
10 92 95
12 93 97
Table 2
(Dissolution in hours pH 6.8 phosphate buffer, 900ml, 50 RPM, Paddle)
The assay (Table 3) for levetiracetam suspension at different time periods shows that the suspension provided an assay without a significant change.
Table 3
The dissolution data (Table 4) for levetiracetam sustained release suspension at different time periods shows that there is no significant change in dissolution of 40°C/75%RH, 1 , 2, 3 Months as compared to the initial data.
Time (Hrs) Dissolution (% Release)
Initial 1 M 2 M 3 M
40°C/75%RH 40°C/75%RH 40°C/75%RH
1 16 18 18 15
2 47 36 36 41
3 62 57 57 58
4 74 68 68 68
6 86 80 80 78
8 90 86 86 84
10 92 90 90 88
12 93 93 93 90
Table 4
(Dissolution in hours pH 6.8 phosphate buffer, 900ml, 50 RPM, Paddle) Example 2
Levetiracetam sustained release suspension for 100 mg/ml
Ingredients Qty in mg
Seal Coating
Sugar Spheres (#60-80) 45.45
Ethyl Cellulose 10 cps 3.64
Purified Talc 0.91
Methyl Alcohol q.s.
MDC q.s.
Levetiracetam Drug Coating
Seal coated pellets 50
Levetiracetam 100
HPMC 6 cps 18.8
Talc 6.2
Methanol q.s.
Extended release coating
Drug coated pellets 175.00
Ethyl Cellulose 45 cps 45.29
HPMC 2910 6 cps 4.53
DEP 4.53
TEC 2.26
Talc 4.53
Ferric oxide yellow 0.11
Methanol q.s.
Methylene chloride q.s.
Acid soluble Coating
EC coated pellets 236.25
Eudragit E- 100 25.74
PEG 6000 5.15
Talc 12.87
Purified water q.s.
IPA q.s.
Acetone q.s.
Preparation of Aqueous suspension
Eudragit E-100 coated pellets 280.00
Purified Water 456.08
Xanthum Gum 4.26
Methyl Paraben 0.61
Propyl Paraben 0.61
Hydrogenated Castor Oil 1.22
Aerosil 6.08
Aspartame 12.16
Sucrose 121.62
Propyl gallate 1.22
Lake of Panceau 1.22
Strawberry 3.04
Suspension with pellets 888.1 1
1 Seal coating: Sifted the sugar pellets through the 60/80 mesh, ethylcellulose (EC) and talc are dispersed in a blend of Methanol/Dichloromethane (DCM). This dispersion was coated on spheres using FBC bottom spray to get seal coated pellets.
2 Drug layering: Dissolved HPMC 6 cps in methanol then added Levetiracetam and Talc to this dispersion, stirred the dispersion to get homogeneous suspension. Seal coated pellets were coated with this suspension using FBC bottom spray to get drug loaded pellets.
3 Extended release layering: Dispersed Diethyl phthatate (DEP), Triethyl citrate (TEC), HPMC 6 cps and Ethyl cellulose (EC) 45 cps in methanol with stirring to get uniform dispersion. Methylene chloride was added to this dispersion to solubilize all dispersed material by stirring. Talc and Ferric oxide yellow are mixed with stirring. Drug loaded pellets were coated with this suspension using FBC bottom spray to get extended release drug pellets.
4 Protective layering: Eudragit and PEG were dissolved in a blend of Purified water/Acetone/ Isopropyl alcohol with stirring. Finally talc was dispersd in this solution with stirring. Extended release drug pellets were coated with this solution using FBP bottom spray.
5 Suspension vehicle preparation: Hydrogenated castor oil, aerosol,xanthan gum with purified water was milled in colloid mill. Continue the milling and added propyl gallate, methyl paraben, propyl paraben and sucrose/asparmate. Finally the color (Lake of Panceau) and flavors (strawberry) are milled. Filter this milled suspension through # 40 sieve.
6. Preparation of Suspension: Mixed the drug pellets of step 4 in suspension vehicle of step 5 with stirring.
Example 3
Ingredients Qty in mg
Seal Coating
Microcrystalline cellulose pellets (#70- 140) 714.285
Ethyl cellulose STD 45 CPS 35.715
Methanol qs
Methylene chloride qs
Drug Loading
Microcrystalline cellulose pellets ( # 60- 100) 750.00
Levetiracetam 500.00
HPMC 6 CPS 62.50
Talc 30.625
Purified water qs
Sustained Release Coating
Drug loaded pellets of Levetiracetam 1343.125
Ethyl cellulose STD 45 CPS 155.80
Diethyl Phthalate 32.20
Talc 9.92
Methanol qs
Methylene chloride qs
Protective coating
Ethyl Cellulose coated Levetiracetam pellets 1541.045
Eudragit E 100 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Total 1760.00
Suspension
Levetiracetam extended release granules 1760.00
Sucralose 100.00
Propylene Glycol 13.28
Glycerin 1.33
Sorbitol Soln (Non crystalliziing) 2.66
Citric acid monohydrate 1.5
Sodium bicarbonate 50.00
Methyl Paraban 5.0
Propyl Paraban 1.0
Selecta Banana Flavour (844439) 1.1
Purified Water q.s
Total 1934.87
Manufacturing Process
Drug loading
1. Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
2. Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
3. Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
Protective coating
4. Sustained release pellets obtained as above are again coated with the dispersion obtained by adding Eudragit E 100, talc, diethyl phthalate in a mixture of isopropyl alcohol and acetone.
5. Above pellets are suspended in purified water along with Sucralose, Propylene Glycol, Glycerin, Sorbitol soln (Non crystallizing), Citric acid monohydrate, Sodium bicarbonate, Methyl Paraben, Propyl Paraben, Selecta Banana Flavour (844439) and filled in bottle for ready to use suspension. Example 4: Sustained release suspension of Levetiracetam by hydrogenated castor oil.
Ingredients Qty in mg
Seal Coating
Microcrystalline Cellulose 714.285
Ethyl cellulose STD 45 CPS 35.715
Methanol qs
Methylene chloride qs
Drug Coating
Seal coated Microcrystalline cellulose pellets 750.00
Levetiracetam 500.00
HPMC 6 CPS 62.50
Talc 30.625
Purified water qs
Polymer Coating
Drug loaded pellets of Levetiracetam 1343.125
Ethyl cellulose STD 45 CPS 155.80
Diethyl Phthalate 32.20
Talc 9.92
Methanol qs
Methylene chloride qs
Hydrophobic Coating
Ethyl Cellulose coated Levetiracetam pellets 1541.045
Hydrogenated castor oil 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Acid soluble Coating
Ethyl Cellulose coated Levetiracetam pellets 1760.00
Eudragit E 100 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Total 1978.955
Manufacturing Process:
Drug loading:
1. Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
2. Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
3. Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
4. Ethyl cellulose pellets are further coated with hydrogenated castor oil.
Acid soluble Coating:
5. Further the pellets obtained from the above step are coated with alkaline soluble polymer Eudragit E 100.
The pellets thus obtained are suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
Example -5: Sustained release liquid dosage form having alkali soluble coating and acid soluble coating.
Ingredients Qty in mg
Seal Coating
Microcrystalline Cellulose pellets 714.285
Ethyl cellulose STD 45 CPS 35.715
Methanol qs
Methylene chloride qs
Drug Coating
Seal coated Microcrystalline cellulose pellets 750.00
Levetiracetam 500.00
HPMC 6 CPS 62.50
Talc 30.625
Purified water qs
Polymer coating
Drug loaded pellets of Levetiracetam 1343.125
Eudragit RSPO 255.80
Diethyl Phthalate 32.20
Talc 9.92
Methanol qs
Methylene chloride qs
Alkali Soluble Coating
Eudragit RSPO coated Levetiracetam pellets 1641.045
Eudragit L 100 -55 124.277
Diethyl Phthalate 22÷968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Acid Soluble Coating
Ethyl Cellulose coated Levetiracetam pellets 1860.0
Eudragit E 100 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Total 2078.955
Drug loading:
1. Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
2. Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
3. Drug loaded pellets are coated with dispersion of Eudragit RSPO obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
Alkali and Acid soluble Coating:
4. Eudragit RSPO pellets are further coated with an alkaline soluble coating comprising of Eudragit L 100-55.
5. Further the pellets obtained from the above step are coated with acid soluble polymer Eudragit E 100.
The pellets thus obtained are suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
Example -6: Sustained release liquid suspension having dual drug release.
Ingredients Qty in mg
Seal Coating
Microcrystalline Cellulose pellets 714.285
Ethyl cellulose STD 45 CPS 35.715
Methanol qs
Methylene chloride qs
Drug Coating
Seal coated Microctystalline cellulose pellets 750.00
Levetiracetam 400.00
HPMC 6 CPS 62.50
Talc 30.625
Purified water qs
Polymer Coating
Drug loaded pellets of Levetiracetam 1243.125
Ethyl cellulose STD 45 CPS 155.80
Diethyl Phthalate 32.20
Talc 9.92
Methanol qs
Methylene chloride qs
Second Drug Coating
Ethyl cellulose coated pellets . 1441.045
Levetiracetam 100.00
HPMC 6 CPS 20.0
Talc 30.0
Purified water qs.
Alkali Soluble Coating
Ethyl Cellulose followed by drug coated pellets 1591.045
Eudragit L 100 -55 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Acid Soluble Coating
Eudragit L 100 -55 coated Levetiracetam pellets . 1810.00
Eudragit E l OO 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Manufacturing Process:
Drug loading:
1. Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
2. Drug binder dispersion is prepared by adding levetiracetam with mixtures of HPMC, talc in water and coated over the seal-coated pellets and further dried.
3. Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
Drug Coating:
4. Drug-binder solution obtained by dissolving levetiracetam, HPMC, talc in purified water is sprayed upon the sustained release pellets obtained above.
5. Further the pellets obtained from the above step are coated with alkaline soluble polymer Eudragit L 100-55.
6. The pellets obtained above are further coated with acid-soluble coating comprising of Eudragit E 100.
The pellets thus obtained are suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
Example -7: Sustained release suspension prepared by ion-exchange resin complexation.
Ingredients Qty in mg
Seal Coating
Microcrystalline Cellulose pellets 714.285
Ethyl cellulose STD 45 CPS 35.715
Methanol qs
Methylene chloride qs
Drug-Resin Complex Formation
Seal coated Microcrystalline cellulose pellets 750.00
Levetiracetam 500.00
Sodium polystyrene sulphonate (Amberlite IR69) 200.00
HPMC 6 CPS 62.50
Talc 30.625
Purified water QS
Polymer coating
Drug - resign loaded pellets of Levetiracetam 1543.125
Ethyl cellulose STD 45 CPS 55.80
Diethyl Phthalate 32.20
Talc 9.92
Methanol qs
Methylene chloride qs
Protective coating
Ethyl Cellulose coated Levetiracetam pellets 1641.045
Eudragit E 100 124.277
Diethyl Phthalate 22.968
Talc 71.71
Isopropyl alcohol qs
Acetone qs
Manufacturing Process:
Drug loading:
1. Ethyl cellulose is dissolved in the mixture of methanol and methylene chloride and sprayed upon microcrystalline cellulose to obtain seal coated pellets.
2. Drug-resin complex solution is prepared by dissolving levetiracetam, ion-exchange resin, talc and HPMC in purified water. The drug-resin complex binder dispersion was sprayed upon the seal coated pellets.
3. Drug loaded pellets are coated with dispersion of ethyl cellulose obtained by adding ethyl cellulose, diethyl phthalate and talc in mixtures of methanol and methylene chloride.
Protective coating:
4. The pellets obtained above are further coated with acid-soluble protective coating comprising of Eudragit E 100.
The pellets thus obtained can be suspended in an aqueous or oily based dispersing agents containing other additives such as sweeteners, flavors, stabilizers, pH modifiers and suspending agent.
Claims
1. A ready to use, stable sustained release liquid oral suspension dosage form comprising: sustained release pellets, said sustained release pellets comprising:
a) Inert pellets, surrounded by seal coating,
b) Drug layer comprising pharmaceutically active ingredient with one or more pharmaceutically acceptable excipients surrounding said seal coated inert pellets, c) Coating layer comprising rate controlling polymer surrounding said drug layer, such that the sustained release pellets are suspended with suitable suspending agent, in addition to other pharmaceutically acceptable excipients in a suspending media at a suitable pH.
2. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 , wherein the Sustained release pellets are further surrounded by drug-binder solution.
3. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 or 2, wherein the Sustained release pellets are further surrounded by protective coating.
4. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in any of preceding claim, wherein the suspending media is an aqueous media.
5. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in any of preceding claim, wherein the suspending media is a non-aqueous media.
6. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in any of preceding claim, has a pH in a range of 6 to 7.5.
7. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1, wherein the drug layer comprises drug resin complex with one or more pharmaceutically acceptable excipient
8. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 , wherein the pharmaceutically active ingredient is a high solubility active ingredient.
9. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 , wherein the pharmaceutically active ingredient is selected from the group comprising of analgesic; anti-allergic; anti-anginal; antibacterial; anticonvulsant; antipsychotic; antidepressant; antidiabetic; anti-epileptic; hypolipidemic; antihyperlipidemic; hypocholesterolemic; antihypertensive; vasoconstrictor; vasodilator; anti-inflammatory; antineoplastic, antiparasitic; antiproliferative; antisecretory; antithrombotic; anti-ulcerative; antiviral; appetite suppressant; diagnostic aid; diuretic; glucocorticoid; immunizing agent; immunomodulator; immunoregulator; immunostimulant; immunosuppressant; neuroprotective; NMDA antagonist; radioactive agent; sedative; sedative-hypnotic; steroid; tranquilizer; cerebral ischemia agent; wound healing agent and xanthine oxidase inhibitor.
10. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 , wherein the pharmaceutically active ingredient is selected from to the group comprising of aspirin, paracetamol, diclofenac, naproxen, oxycodone, hydrocodone, diamorphine, carbamazepine, topiramate, lamotrigine, Levetiracetam, Brivaracetam, seletracetam, primidone, clonazepam, diazepam, oxcarbazepine, zonisamide, pregabalin, ethosuximide, lorazepam, nitrazepam, midazolam, lorazepam, tolbutamide, acetohexamide, tolazamide, chlo ropamide, metoprolol, metformin, phenformin, buformin, rosiglitazone, pioglitazone,troglitazone, miglitol, acarbose, alogliptin, vildagliptin, sitagliptin, saxagliptin, glipizide, glimeperide, glyburide, repaglinide, nateglinide, Fluphenazine ,Perphenazine,
ΡΓθθηΙο βΓβζΐηε, Thioridazine, Trifluoperazine, Mesoridazine, Periciazine, Promazine, Triflupromazine, Levomepromazine, Promethazine, Pimozide chlorprothixene, clopenthixol, flupenthixol, thithixene, Zuclopenthixol, Clozapine, Olanzapine, Risperidone, Paliperidone, Quetiqpine, Ziprasidone, Amisulpride, Asenapine, Iloperidone, Zotepine, Sertindole, Lurasidone, Aripiprazole, alprazolam,
Zolpidem, bupropion, duloxetine, milnacipran, tolterodine, diltiazem, Oxybutinin, Tramadol, Tapentadol, venlafaxine, desvenlafaxine or there pharmaceutical acceptable salts and a combination thereof.
1 1. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 1 , wherein the pharmaceutically active ingredient is levetiracetam.
12. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in claim 1 , wherein the pharmaceutically acceptable excipients are selected from the group of fillers, binders, lubricants, glidants, rate controlling polymers, suspension stabilizers, buffering agents, sweeteners, antioxidants, flavoring agents, viscosity modifying agent, surfactants, preservatives, humectants, plasticizers and resins.
13. A ready to use, stable sustained release liquid oral suspension dosage form as claimed in any of preceding claim, wherein it does not require reconstitution before oral administration. A process for preparation of ready to use, stable sustained release liquid oral suspension dosage form comprising the steps:
a) coating suitable fillers with a binder solution to obtain seal coated inert pellets;
b) further coating said seal coated inert pellets obtained from step a) with a drug- binder solution or drug-binder resin complex;
c) further coating drug coated pellets so obtained from step b) with a rate controlling polymer with one or more pharmaceutically acceptable excipients; d) Optionally, further coating the pellets so obtained in step c with drug binder solution or drug resin complex;
e) Applying the protective coating to the the pellets so obtained in step c) or d) using suitable polymer
f) forming suspension of the sustained release pellets so obtained using suitable suspending agent along with sweeteners, flavoring agent, buffering agent, preservative, humectants in addition to other pharmaceutically acceptable excipients in aqueous or non aqueous media at a pH being maintained with or without buffer.
Applications Claiming Priority (2)
| Application Number | Priority Date | Filing Date | Title |
|---|---|---|---|
| IN577/MUM/2010 | 2010-03-04 | ||
| IN577MU2010 | 2010-03-04 |
Publications (2)
| Publication Number | Publication Date |
|---|---|
| WO2011107855A2 true WO2011107855A2 (en) | 2011-09-09 |
| WO2011107855A3 WO2011107855A3 (en) | 2012-03-29 |
Family
ID=44202596
Family Applications (1)
| Application Number | Title | Priority Date | Filing Date |
|---|---|---|---|
| PCT/IB2011/000426 WO2011107855A2 (en) | 2010-03-04 | 2011-03-02 | Sustained release oral liquid suspension dosage form |
Country Status (1)
| Country | Link |
|---|---|
| WO (1) | WO2011107855A2 (en) |
Cited By (40)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| CN103432128A (en) * | 2013-08-26 | 2013-12-11 | 中国人民解放军第150中心医院 | Method for compounding drug-containing layer of pioglitazone hydrochloride controlled-release pellet preparation |
| CN103462922A (en) * | 2012-06-06 | 2013-12-25 | 南京亿华药业有限公司 | Levetiracetam sustained release tablet and preparation method thereof |
| US8652527B1 (en) | 2013-03-13 | 2014-02-18 | Upsher-Smith Laboratories, Inc | Extended-release topiramate capsules |
| CN103966680A (en) * | 2014-05-04 | 2014-08-06 | 东华大学 | Method for preparing drug sustained release nanofibers |
| US20140356314A1 (en) * | 2013-05-31 | 2014-12-04 | Arevipharma Gmbh | 3-[3-(dimethylamino)-1-ethyl-2-methylpropyl]phenol resin complex |
| EP2829267A1 (en) * | 2012-03-23 | 2015-01-28 | Institute of Pharmacology and Toxicology Academy of Military Medical Sciences P.L.A. China | Topiramate sustained-release pharmaceutical composition, method for preparing same, and use thereof |
| US20150051289A1 (en) * | 2012-02-28 | 2015-02-19 | Ammtek | Liquid formulations of hypoglycaemic sulfonamides |
| US9101545B2 (en) | 2013-03-15 | 2015-08-11 | Upsher-Smith Laboratories, Inc. | Extended-release topiramate capsules |
| WO2016010741A1 (en) * | 2014-07-17 | 2016-01-21 | Dow Global Technologies Llc | Ethylcellulose dispersion and film |
| US20160228360A1 (en) * | 2014-05-01 | 2016-08-11 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of metformin |
| US9421188B2 (en) | 2012-03-23 | 2016-08-23 | Institute Of Pharmacology And Toxicology Academy Of Military Medical Sciences P.L.A. China | Combination product comprising phentermine and topiramate, and preparation method thereof |
| US9457008B2 (en) | 2012-03-23 | 2016-10-04 | Institute Of Pharmacology And Toxicology Academy Of Military Medical Sciences P.L.A. China | Joint product comprising synephrine and topiramate |
| US9492444B2 (en) | 2013-12-17 | 2016-11-15 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| WO2017025930A1 (en) | 2015-08-12 | 2017-02-16 | Ftf Pharma Private Limited | Oral solution of aripiprazole |
| US9707184B2 (en) | 2014-07-17 | 2017-07-18 | Pharmaceutical Manufacturing Research Services, Inc. | Immediate release abuse deterrent liquid fill dosage form |
| US20180008539A1 (en) * | 2014-12-05 | 2018-01-11 | Sun Pharmaceutical Industries Limited | Gastroretentive extended release suspension compositions |
| CN107669624A (en) * | 2017-09-28 | 2018-02-09 | 健民集团叶开泰国药(随州)有限公司 | A kind of Levetiracetam oral administration solution and preparation method thereof |
| US9962336B2 (en) | 2014-05-01 | 2018-05-08 | Sun Pharmaceutical Industries Limited | Extended release suspension compositions |
| US9962345B2 (en) | 2014-05-01 | 2018-05-08 | Sun Pharmaceutical Industries Limited | Oral liquid compositions of guanfacine |
| CN108348475A (en) * | 2015-07-17 | 2018-07-31 | 比利时法贝尔制造股份有限公司 | Multilayer pharmaceutically active compound release microparticles in liquid dosage form |
| US10172797B2 (en) | 2013-12-17 | 2019-01-08 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| US10195153B2 (en) | 2013-08-12 | 2019-02-05 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded immediate release abuse deterrent pill |
| EP3441064A1 (en) * | 2017-08-11 | 2019-02-13 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of guaifenesin |
| US10238803B2 (en) | 2016-05-02 | 2019-03-26 | Sun Pharmaceutical Industries Limited | Drug delivery device for pharmaceutical compositions |
| US10258583B2 (en) | 2014-05-01 | 2019-04-16 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of guanfacine |
| WO2019074829A1 (en) * | 2017-10-09 | 2019-04-18 | Rhodes Pharmaceuticals L.P. | Pharmaceutical resinate compositions and methods of making and using thereof |
| US10285908B2 (en) | 2014-07-30 | 2019-05-14 | Sun Pharmaceutical Industries Ltd | Dual-chamber pack |
| US10369078B2 (en) | 2016-05-02 | 2019-08-06 | Sun Pharmaceutical Industries Limited | Dual-chamber pack for pharmaceutical compositions |
| WO2020011938A1 (en) | 2018-07-11 | 2020-01-16 | Medizinische Universität Wien | Glucocorticoids for the topical treatment of autoimmune gastritis |
| US20200315978A1 (en) * | 2015-07-17 | 2020-10-08 | BE Pharbel Manufacturing | Multilayered pharmaceutically active compound-releasing microparticles in a liquid dosage form |
| US10898449B2 (en) | 2016-12-20 | 2021-01-26 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine |
| US10959958B2 (en) | 2014-10-20 | 2021-03-30 | Pharmaceutical Manufacturing Research Services, Inc. | Extended release abuse deterrent liquid fill dosage form |
| US11033512B2 (en) | 2017-06-26 | 2021-06-15 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine and silicone acrylic hybrid polymer |
| CN113698384A (en) * | 2021-10-26 | 2021-11-26 | 上海维京生物医药科技有限公司 | Alogliptin gallate and preparation method and application thereof |
| US11337932B2 (en) | 2016-12-20 | 2022-05-24 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine and polysiloxane or polyisobutylene |
| US11504345B2 (en) | 2014-05-01 | 2022-11-22 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of metformin |
| WO2023002004A1 (en) | 2021-07-23 | 2023-01-26 | Laboratorios Lesvi, S.L. | Multiparticulate pharmaceutical composition |
| US11564918B2 (en) | 2016-10-10 | 2023-01-31 | Rhodes Pharmaceuticals L.P. | Pharmaceutical resinate compositions and methods of making and using thereof |
| US11648213B2 (en) | 2018-06-20 | 2023-05-16 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine |
| US11660272B2 (en) | 2017-10-12 | 2023-05-30 | University Of Hertfordshire Higher Education Corporation | Method for coating particles |
Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US4999189A (en) | 1988-11-14 | 1991-03-12 | Schering Corporation | Sustained release oral suspensions |
| US5156842A (en) | 1987-06-19 | 1992-10-20 | Elan Corporation, Plc | Liquid suspension for oral administration |
| US5296236A (en) | 1988-09-16 | 1994-03-22 | Recordati S.A., Chemical And Pharmaceutical Company | Controlled release therapeutic system for a liquid pharmaceutical formulations |
| EP0717992A2 (en) | 1994-12-21 | 1996-06-26 | McNEIL-PPC, INC. | Aqueous suspension formulations for pharmaceutical applications |
| US6156340A (en) | 1996-03-29 | 2000-12-05 | Duquesne University Of The Holy Ghost | Orally administrable time release drug containing products |
| US20030108575A1 (en) | 2001-08-06 | 2003-06-12 | Lu Guang Wei | Stabilized oral suspension formulation |
| WO2003053403A2 (en) | 2001-12-20 | 2003-07-03 | Pharmacia Corporation | Pharmaceutical suspension for oral administration |
| WO2006093838A2 (en) | 2005-02-28 | 2006-09-08 | Neos Therapeutics, Lp | Compositions and methods of making sustained release liquid formulations |
| US7300670B2 (en) | 2002-04-03 | 2007-11-27 | Unilab Pharmatech, Ltd. | Oral suspension formulation |
| WO2008122993A1 (en) | 2007-04-09 | 2008-10-16 | Panacea Biotec Limited | Controlled release formulation of coated microparticles |
| US20090111872A1 (en) | 2007-10-31 | 2009-04-30 | Roger EMBRECHTS | Stabilized pediatric suspension of carisbamate |
Family Cites Families (6)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20050191353A1 (en) * | 2002-08-16 | 2005-09-01 | Amit Krishna Antarkar | Process for manufacture of stable oral multiple unit pharmaceutical composition containing benzimidazoles |
| DE10353196A1 (en) * | 2003-11-13 | 2005-06-16 | Röhm GmbH & Co. KG | Multilayer dosage form with a matrix influencing the delivery of a modulatory substance |
| WO2006011159A2 (en) * | 2004-06-21 | 2006-02-02 | Torrent Pharmaceuticals Limited | Stabilized pharmaceutical composition containing rabeprazole sodium with improved bioavailability |
| US20090175935A1 (en) * | 2006-08-14 | 2009-07-09 | Torrent Research Ltd. | Pharmaceutical compositions of duloxetine |
| JP2010024156A (en) * | 2008-07-16 | 2010-02-04 | Ucb Pharma Sa | Pharmaceutical composition comprising levetiracetam |
| PL2358360T3 (en) * | 2008-11-18 | 2017-02-28 | Ucb Biopharma Sprl | Prolonged release formulations comprising an 2-oxo-1-pyrrolidine derivative |
-
2011
- 2011-03-02 WO PCT/IB2011/000426 patent/WO2011107855A2/en active Application Filing
Patent Citations (11)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US5156842A (en) | 1987-06-19 | 1992-10-20 | Elan Corporation, Plc | Liquid suspension for oral administration |
| US5296236A (en) | 1988-09-16 | 1994-03-22 | Recordati S.A., Chemical And Pharmaceutical Company | Controlled release therapeutic system for a liquid pharmaceutical formulations |
| US4999189A (en) | 1988-11-14 | 1991-03-12 | Schering Corporation | Sustained release oral suspensions |
| EP0717992A2 (en) | 1994-12-21 | 1996-06-26 | McNEIL-PPC, INC. | Aqueous suspension formulations for pharmaceutical applications |
| US6156340A (en) | 1996-03-29 | 2000-12-05 | Duquesne University Of The Holy Ghost | Orally administrable time release drug containing products |
| US20030108575A1 (en) | 2001-08-06 | 2003-06-12 | Lu Guang Wei | Stabilized oral suspension formulation |
| WO2003053403A2 (en) | 2001-12-20 | 2003-07-03 | Pharmacia Corporation | Pharmaceutical suspension for oral administration |
| US7300670B2 (en) | 2002-04-03 | 2007-11-27 | Unilab Pharmatech, Ltd. | Oral suspension formulation |
| WO2006093838A2 (en) | 2005-02-28 | 2006-09-08 | Neos Therapeutics, Lp | Compositions and methods of making sustained release liquid formulations |
| WO2008122993A1 (en) | 2007-04-09 | 2008-10-16 | Panacea Biotec Limited | Controlled release formulation of coated microparticles |
| US20090111872A1 (en) | 2007-10-31 | 2009-04-30 | Roger EMBRECHTS | Stabilized pediatric suspension of carisbamate |
Cited By (60)
| Publication number | Priority date | Publication date | Assignee | Title |
|---|---|---|---|---|
| US20150051289A1 (en) * | 2012-02-28 | 2015-02-19 | Ammtek | Liquid formulations of hypoglycaemic sulfonamides |
| US11911505B2 (en) | 2012-02-28 | 2024-02-27 | Ammtek | Liquid formulations of hypoglycaemic sulfonamides |
| US11110059B2 (en) * | 2012-02-28 | 2021-09-07 | Ammtek | Liquid formulations of hypoglycaemic sulfonamides |
| US9421188B2 (en) | 2012-03-23 | 2016-08-23 | Institute Of Pharmacology And Toxicology Academy Of Military Medical Sciences P.L.A. China | Combination product comprising phentermine and topiramate, and preparation method thereof |
| EP2829267A4 (en) * | 2012-03-23 | 2015-09-30 | Inst Pharm & Toxicology Amms | Topiramate sustained-release pharmaceutical composition, method for preparing same, and use thereof |
| US9457008B2 (en) | 2012-03-23 | 2016-10-04 | Institute Of Pharmacology And Toxicology Academy Of Military Medical Sciences P.L.A. China | Joint product comprising synephrine and topiramate |
| EP2829267A1 (en) * | 2012-03-23 | 2015-01-28 | Institute of Pharmacology and Toxicology Academy of Military Medical Sciences P.L.A. China | Topiramate sustained-release pharmaceutical composition, method for preparing same, and use thereof |
| CN103462922A (en) * | 2012-06-06 | 2013-12-25 | 南京亿华药业有限公司 | Levetiracetam sustained release tablet and preparation method thereof |
| CN103462922B (en) * | 2012-06-06 | 2016-01-20 | 南京亿华药业有限公司 | A kind of levetiracetam sustained-release tablets and method for making thereof |
| US10363224B2 (en) | 2013-03-13 | 2019-07-30 | Upsher-Smith Laboratories, Llc | Extended-release topiramate capsules |
| US8889190B2 (en) | 2013-03-13 | 2014-11-18 | Upsher-Smith Laboratories, Inc. | Extended-release topiramate capsules |
| US8652527B1 (en) | 2013-03-13 | 2014-02-18 | Upsher-Smith Laboratories, Inc | Extended-release topiramate capsules |
| US9101545B2 (en) | 2013-03-15 | 2015-08-11 | Upsher-Smith Laboratories, Inc. | Extended-release topiramate capsules |
| US10172878B2 (en) | 2013-03-15 | 2019-01-08 | Upsher-Smith Laboratories, Llc | Extended-release topiramate capsules |
| US9555005B2 (en) | 2013-03-15 | 2017-01-31 | Upsher-Smith Laboratories, Inc. | Extended-release topiramate capsules |
| US20140356314A1 (en) * | 2013-05-31 | 2014-12-04 | Arevipharma Gmbh | 3-[3-(dimethylamino)-1-ethyl-2-methylpropyl]phenol resin complex |
| US10639281B2 (en) | 2013-08-12 | 2020-05-05 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded immediate release abuse deterrent pill |
| US10195153B2 (en) | 2013-08-12 | 2019-02-05 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded immediate release abuse deterrent pill |
| CN103432128B (en) * | 2013-08-26 | 2015-11-18 | 中国人民解放军第150中心医院 | A kind of compound formulation of pioglitazone hydrochloride sustained-release pellet preparations medicated layer |
| CN103432128A (en) * | 2013-08-26 | 2013-12-11 | 中国人民解放军第150中心医院 | Method for compounding drug-containing layer of pioglitazone hydrochloride controlled-release pellet preparation |
| US9492444B2 (en) | 2013-12-17 | 2016-11-15 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| US10792254B2 (en) | 2013-12-17 | 2020-10-06 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| US10172797B2 (en) | 2013-12-17 | 2019-01-08 | Pharmaceutical Manufacturing Research Services, Inc. | Extruded extended release abuse deterrent pill |
| EP3137060B1 (en) | 2014-05-01 | 2020-11-11 | Sun Pharmaceutical Industries Ltd | Extended release suspension compositions |
| US11504345B2 (en) | 2014-05-01 | 2022-11-22 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of metformin |
| US9962336B2 (en) | 2014-05-01 | 2018-05-08 | Sun Pharmaceutical Industries Limited | Extended release suspension compositions |
| US9962345B2 (en) | 2014-05-01 | 2018-05-08 | Sun Pharmaceutical Industries Limited | Oral liquid compositions of guanfacine |
| US20160228360A1 (en) * | 2014-05-01 | 2016-08-11 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of metformin |
| US11523996B2 (en) | 2014-05-01 | 2022-12-13 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of metformin |
| US10258583B2 (en) | 2014-05-01 | 2019-04-16 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of guanfacine |
| CN103966680A (en) * | 2014-05-04 | 2014-08-06 | 东华大学 | Method for preparing drug sustained release nanofibers |
| US9707184B2 (en) | 2014-07-17 | 2017-07-18 | Pharmaceutical Manufacturing Research Services, Inc. | Immediate release abuse deterrent liquid fill dosage form |
| CN106488778A (en) * | 2014-07-17 | 2017-03-08 | 陶氏环球技术有限责任公司 | Ethylcellulose dispersion and thin film |
| WO2016010741A1 (en) * | 2014-07-17 | 2016-01-21 | Dow Global Technologies Llc | Ethylcellulose dispersion and film |
| US10285908B2 (en) | 2014-07-30 | 2019-05-14 | Sun Pharmaceutical Industries Ltd | Dual-chamber pack |
| US10959958B2 (en) | 2014-10-20 | 2021-03-30 | Pharmaceutical Manufacturing Research Services, Inc. | Extended release abuse deterrent liquid fill dosage form |
| US20180008539A1 (en) * | 2014-12-05 | 2018-01-11 | Sun Pharmaceutical Industries Limited | Gastroretentive extended release suspension compositions |
| EP3324948B1 (en) * | 2015-07-17 | 2023-02-15 | BE Pharbel Manufacturing | Multilayered pharmaceutically active compound-releasing microparticles in a liquid dosage form |
| JP2018521137A (en) * | 2015-07-17 | 2018-08-02 | ビーイー ファーベル マニュファクチャリング | Pharmaceutically active compound releasing multilayer microparticles in liquid dosage forms |
| CN108348475A (en) * | 2015-07-17 | 2018-07-31 | 比利时法贝尔制造股份有限公司 | Multilayer pharmaceutically active compound release microparticles in liquid dosage form |
| US20200315978A1 (en) * | 2015-07-17 | 2020-10-08 | BE Pharbel Manufacturing | Multilayered pharmaceutically active compound-releasing microparticles in a liquid dosage form |
| WO2017025930A1 (en) | 2015-08-12 | 2017-02-16 | Ftf Pharma Private Limited | Oral solution of aripiprazole |
| US10238803B2 (en) | 2016-05-02 | 2019-03-26 | Sun Pharmaceutical Industries Limited | Drug delivery device for pharmaceutical compositions |
| US10369078B2 (en) | 2016-05-02 | 2019-08-06 | Sun Pharmaceutical Industries Limited | Dual-chamber pack for pharmaceutical compositions |
| US11564918B2 (en) | 2016-10-10 | 2023-01-31 | Rhodes Pharmaceuticals L.P. | Pharmaceutical resinate compositions and methods of making and using thereof |
| US11337932B2 (en) | 2016-12-20 | 2022-05-24 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine and polysiloxane or polyisobutylene |
| US10898449B2 (en) | 2016-12-20 | 2021-01-26 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine |
| US10980753B2 (en) | 2016-12-20 | 2021-04-20 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine |
| US11033512B2 (en) | 2017-06-26 | 2021-06-15 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine and silicone acrylic hybrid polymer |
| EP3441064A1 (en) * | 2017-08-11 | 2019-02-13 | Sun Pharmaceutical Industries Limited | Extended release liquid compositions of guaifenesin |
| CN107669624A (en) * | 2017-09-28 | 2018-02-09 | 健民集团叶开泰国药(随州)有限公司 | A kind of Levetiracetam oral administration solution and preparation method thereof |
| WO2019074829A1 (en) * | 2017-10-09 | 2019-04-18 | Rhodes Pharmaceuticals L.P. | Pharmaceutical resinate compositions and methods of making and using thereof |
| CN111629758A (en) * | 2017-10-09 | 2020-09-04 | 罗兹制药公司 | Pharmaceutical resinate compositions and methods of making and using same |
| US11690840B2 (en) | 2017-10-09 | 2023-07-04 | Rhodes Pharmaceuticals L.P. | Pharmaceutical resinate compositions and methods of making and using thereof |
| US11660272B2 (en) | 2017-10-12 | 2023-05-30 | University Of Hertfordshire Higher Education Corporation | Method for coating particles |
| US11648213B2 (en) | 2018-06-20 | 2023-05-16 | Lts Lohmann Therapie-Systeme Ag | Transdermal therapeutic system containing asenapine |
| WO2020011938A1 (en) | 2018-07-11 | 2020-01-16 | Medizinische Universität Wien | Glucocorticoids for the topical treatment of autoimmune gastritis |
| WO2023002004A1 (en) | 2021-07-23 | 2023-01-26 | Laboratorios Lesvi, S.L. | Multiparticulate pharmaceutical composition |
| CN113698384B (en) * | 2021-10-26 | 2022-01-18 | 上海维京生物医药科技有限公司 | Alogliptin gallate and preparation method and application thereof |
| CN113698384A (en) * | 2021-10-26 | 2021-11-26 | 上海维京生物医药科技有限公司 | Alogliptin gallate and preparation method and application thereof |
Also Published As
| Publication number | Publication date |
|---|---|
| WO2011107855A3 (en) | 2012-03-29 |
Similar Documents
| Publication | Publication Date | Title |
|---|---|---|
| WO2011107855A2 (en) | Sustained release oral liquid suspension dosage form | |
| US9962336B2 (en) | Extended release suspension compositions | |
| US8840924B2 (en) | Compositions and methods of making rapidly dissolving ionically masked formulations | |
| KR940000098B1 (en) | Process for preparing pharmaceutical preparation with controlled release of the active compound | |
| AU2018250470A1 (en) | Extended release suspension compositions | |
| US20070141147A1 (en) | Sequential release pharmaceutical formulations | |
| WO2017182851A1 (en) | Extended release suspension compositions | |
| US20090017127A1 (en) | Modified Release Dosage Forms of Skeletal Muscle Relaxants | |
| AU2010277207B2 (en) | Multi-layered, multiple unit pharmaceutical compositions | |
| AU2009259993B2 (en) | Preparation of controlled release skeletal muscle relaxant dosage forms | |
| JPH01502589A (en) | Taste-masked pharmaceutical compositions | |
| US20050152967A1 (en) | Dynamic variable release | |
| US10369078B2 (en) | Dual-chamber pack for pharmaceutical compositions | |
| PT97369B (en) | METHOD FOR PREPARING PHARMACEUTICAL FORMULATIONS IN WHICH THE FLAVOR OF THE MEDICINE AND DISFORCED BY MEANS OF COATING OF THE MEDICINE WITH A POLYMERIC MEMBRANE | |
| CA3023207C (en) | Dual-chamber pack for pharmaceutical compositions | |
| EP1194153A1 (en) | Taste masked pharmaceutical liquid formulations | |
| BRPI0615014A2 (en) | solid pharmaceutical composition comprising 1- (4-chloroanilino) -4- (4-pyridylmethyl) phthalazine and a ph modifier and use thereof | |
| WO2014028878A1 (en) | Pharmaceutical compositions of memantine | |
| EP3501476A1 (en) | Drug delivery device for pharmaceutical compositions | |
| EP2736496B1 (en) | Pharmaceutical composition containing an antimuscarinic agent and method for the preparation thereof | |
| CA2989925C (en) | Drug delivery device for pharmaceutical compositions | |
| JP2023017052A (en) | Drug delivery device for pharmaceutical compositions | |
| US20160158212A1 (en) | Extended release aqueous suspension of methylphenidate or salts thereof | |
| AU2017279809A1 (en) | Drug delivery device for pharmaceutical compositions | |
| US20160166702A1 (en) | Extended release aqueous suspension of methylphenidate or salts thereof |
Legal Events
| Date | Code | Title | Description |
|---|---|---|---|
| 121 | Ep: the epo has been informed by wipo that ep was designated in this application |
Ref document number: 11715736 Country of ref document: EP Kind code of ref document: A2 |
|
| NENP | Non-entry into the national phase in: |
Ref country code: DE |
|
| WWE | Wipo information: entry into national phase |
Ref document number: 2138/MUMNP/2012 Country of ref document: IN |
|
| 122 | Ep: pct application non-entry in european phase |
Ref document number: 11715736 Country of ref document: EP Kind code of ref document: A2 |