SPHERICAL PARTICLES PRODUCED BY A HOT-MELT EXTRUSION/SPHERONIZATION PROCESS
INVENTORS:
Christopher R. Young, John J. Koleng, and James W. McGinity FIELD OF THE INVENTION
The present invention concerns a combination process for spheronization of a formulation. More particularly, the invention concerns a process wherein a hot-melt extruded solid substrate, such as a pellet, is spheronized.
BACKGROUND OF THE INVENTION Drug layering and wet-mass extrusion/spheronization, also known as granulation/spheronization, is the conventional combination process used for spherical pellet production. Drag layering uses a fluid-bed coater to apply a drug containing film onto non-pareil beads. A non-pareil bead is one that contains no active agent. The disadvantage of this method is the difficulty of calculating the amount of drug added since coating solution is lost onto the coating apparatus. In addition, content uniformity of the layered beads is often a problem. In wet-mass granulation/spheronization a solvent and/or binder solution is added to a dry powder blend to form a wet mass. The wet mass is then extruded without melting of the mass to form a granule that is then spheronized. This technique is disadvantageous because it employs water or other solvents that require a time-consuming drying step. These solvents may degrade the active agent in a formulation, and in practice, it is difficult to remove all traces of solvent from a spheronized granule made according to the wet method. Some solvents are also potentially dangerous for plant workers and for the environment. Furthermore, the use of solvents is expensive because manufacturers must remove and account for all solvents used, and because they must pay for proper solvent disposal.
Hot-melt extrusion, a widely applied processing technique used in the plastics industry, is becoming a viable technique for use in the pharmaceutical industry. This method can be used to prepare granules, sustained-release tablets, and transdermal drug delivery systems. Hot-melt extrusion is rapidly gaining popularity in the pharmaceutical industry because it provides several advantages over traditional production techniques. To
date, however, no process combining the steps of hot-melt extrusion and spheronization has been developed.
Thus, none of the known methods for the formation of spherical pellets, e.g., spherical particles or microspheres, employs a solvent-free process that combines hot-melt extrusion and spheronization, and the known methods are therefore subject to the above- mentioned disadvantages.
SUMMARY OF THE INVENTION
The present invention seeks to overcome some or all of the disadvantages inherent in the above-mentioned wet-mass granulation/spheronization combination process. The present invention provides a process for producing spheronized pellets by hot-melt extrusion and spheronization. The process described herein does not require the addition of a solvent, thus reducing processing times and eliminating other problems associated with solvent addition. Furthermore, the process can be run continually or semi- continually thereby reducing down time in the manufacture of the spheronized pellets. The process also produces spherical pellets having a narrow particle size distribution, and the pellets also provide a greater control of drag release when compared to spheronized pellets made according to a conventional wet-mass granulation/spheronization process derived from the same composition. A drug formulation that is a rapid or immediate release formulation when prepared by wet-mass granulation/spheronization can be converted to a controlled or extended release formulation if the same drag formulation is instead processed as described herein.
One aspect of the invention provides a spheronized particle made according to a process comprising the steps of: hot-melt extruding a thermoformable composition comprising an active agent and at least one pharmaceutical excipient to form an extruded solid; sizing the extruded solid to form a particle; spheronizing the particle for a sufficient period of time at a temperature sufficient to form the spheronized particle.
Another aspect of the invention provides a combination hot-melt extrusion/spheronization process for preparing a spheronized particle, wherein the process comprises the steps of: hot-melt extruding a thermoformable composition comprising an active agent and at least one pharmaceutical excipient to form an extruded solid; sizing the extraded solid to form a particle; spheronizing the particle for a sufficient period of time at a temperature sufficient to form the spheronized particle.
Yet another aspect of the invention provides a hot-melt extraded/spheronized particle comprising: a hot-melt extruded solid substrate comprising at least one thermoformable material, at least one active agent and optionally a plasticizer; wherein the substrate has been spheronized in the presence of heat.
Still another aspect of the invention provides a method of converting a rapid or immediate release formulation to a controlled or extended release hot-melt extraded/spheronized particle, the method comprising the steps of: hot-melt extruding a formulation comprising at least one thermoformable material, at least one active agent and optionally a plasticizer to form an extraded solid,; and spheronizing the extruded solid in the presence of heat to form a controlled or extended release hot-melt extraded/spheronized particle; wherein the formulation instead provides a rapid or immediate release solid when prepared by wet-mass granulation/spheronization.
Specific embodiments of the invention include those wherein: 1) the active agent is a pharmaceutical agent; 2) the composition comprises a thermoformable material that melts or softens during hot-melt extrusion; 3) the extraded solid is cooled prior to sizing; 4) the extruded solid is sized immediately after extrasion; 5) the process produces more than one spheronized particle; 6) process is run batch-wise, continuously or semi- continuously; 7) the composition comprises two or more pharmaceutical excipients; 8) at least one of the pharmaceutical excipients, optionally plasticized, has a glass transition temperature (Tg) below the decomposition temperatures of the components in the
composition; 9) the composition further comprises a plasticizer; 10) the extruded particle is sized by chopping, cutting, or grinding; 11) the sized particles is less than about 10 mm, or is about 0.1-5 mm, in length or diameter; 12) the temperature for spheronizing the particle approximates the Tg of the thermoformable material after hot-melt extrusion; 13) the spheronized particle has a diameter of about 0.1-5 mm; 14) the process further comprises the step of dusting the particles with a pharmaceutical excipient prior to or during spheronization to minimize sticking of the particles; 15) the composition comprises at least an active agent, a binder and a plasticizer; 16) the step of hot-melt extruding is conducted in an extruder having more than one heating zone; 17) the step of hot-melt extruding is conducted in a single or twin screw extruder; and/or 18) the die is round.
BRIEF DESCRIPTION OF THE FIGURES
The following figures form part of the present description and describe exemplary embodiments of the claimed invention. The skilled artisan will, in light of these figures and the description herein, be able to practice the invention without undue experimentation.
FIG. 1 depicts a general schematic of the combination hot-melt extrusion/spheronization process of the invention.
FIGS. 2A-2B depict electron micrographs of the surface of individual particles prepared by wet mass granulation/spheronization (FIG. 2A) and the combination hot-melt extrusion/spheronization process of the invention (FIG. 2B).
FIGS. 3A-3B depict electron micrographs of the cross-section of the particles of FIG. 2A and FIG. 2B, respectively.
FIG. 4 depicts a particle size distribution curve of the spherical pellets prepared by the process of the invention (■) and a wet mass granulation process (U ). FIG. 5 depicts a release profile for theophylline from spherical pellets in pH 1.2 medium (USP apparatus 2, n=3, 900ml, lOOrpm) as the theophylline is released from wet- mass granulated spherical pellets (•) or hot-melt extruded spherical pellets (■).
FIG. 6 depicts a release profile for theophylline from spherical pellets in pH 3.0 medium (USP apparatus 2, n=3, 900ml, lOOrpm) as the theophylline is released from wet- mass granulated spherical pellets (•) or hot-melt extraded spherical pellets (■).
FIG. 7 depicts a release profile for theophylline from spherical pellets in pH 6.8 medium (USP apparatus 2, n=3, 900ml, lOOrpm) as the theophylline is released from wet- mass granulated spherical pellets (•) or hot-melt extraded spherical pellets (■).
FIG. 8 depicts a release profile for theophylline from spherical pellets in pH 7.4 medium (USP apparatus 2, n=3, 900ml, lOOrpm) as the theophylline is released from wet- mass granulated spherical pellets (•) or hot-melt extraded spherical pellets (■). FIG. 9 depicts a release profile for theophylline as a function of pH of the medium (USP apparatus 3; Biodis, n=3, 900ml, lOOrpm) as the theophylline is released from wet-mass granulated spherical pellets (•) or hot-melt extruded spherical pellets (■).
FIG. 10 depicts the release profiles for theophylline from spherical pellets according to the invention initially (•) and after one year of storage (■) at 30°C/60%RH. FIG. 11 depicts the influence of media pH on the release of theophylline from pellets produced by hot-melt extrusion/spheronization according to Example 7.
FIG. 12 depicts the influence of media pH on the release of theophylline from pellets produced by hot-melt extrusion/spheronization according to Example 2.
FIG. 13 depicts the influence of media pH on the release of theophylline from pellets produced by hot-melt extrusion/spheronization according to Example 11.
FIG. 14 depicts a release profile for theophylline as a function of pH of the medium as the theophylline is released from the hot-melt extraded/spheronized pellets of Example 11.
FIG. 15 depicts the influence of media pH on the release of theophylline from pellets produced by hot-melt extrusion/spheronization according to Example 12.
FIG. 16 depicts the influence of media pH on the release of theophylline from pellets produced by hot-melt extrusion/spheronization according to Example 13.
DETAILED DESCRIPTION OF THE INVENTION
The thermoformable composition used to prepare a spheronized pellet according to the invention generally comprises a mixture comprising an active agent, a pharmaceutical excipient, such as a thermopolymer, and one or more other pharmaceutical excipients. The pharmaceutical excipient, which might or might not be plasticized, melts or softens during the hot-melt extrusion process. Hot-melt extraded/spheronized pellets according to the invention were prepared by cutting a thin, hot-melt, extruded composite rod into symmetrical pellets. The pellets were then spheronized in a traditional spheronizer at elevated temperatures, i.e., temperatures above room temperature, to form the hot-melt extraded/spheronized particles, otherwise referred to as spheronized particles. Unlike wet-mass extraded/spheronized particles, the hot-melt extraded/spheronized pellets having the same initial formulation displayed a narrower particle size distribution. Moreover, the hot-melt extraded/spheronized pellets provided a slower release profile. Accordingly, hot-melt extrasion of a first formulation provided a hot-melt extraded/spheronized solid having a slower overall active agent release rate than a wet- mass extraded/spheronized solid having the same first formulation.
As used herein, a "thermoformable" material or composition refers to one that will soften or melt during the hot-melt extrusion step. The thermoformable material or composition may require a plasticizer to render it thermoformable. As used herein, a "thermopolymer" is generally a polymeric pharmaceutical excipient that melts or softens during the extrasion process. By using a thermopolymer in the composition, a more intimately mixed extraded solid is produced by hot-melt extrasion than is formed by a wet-mass granulation method using the same composition. Consequently, the spherical particle produced by spheronization has a more homogeneous composition, smoother surface and more controlled release of active agent.
Materials used herein are available as follows. EUDRAGIT® Preparation 4135F is a copolymer from Rohm GmbH (Darmstadt, Germany). The copolymer comprises the monomers methacrylic acid, methyl methacrylate and methyl acrylate in a ratio of about 10:25:65. The copolymer has a pH dependent solubility. The preparation is used for colonic (enteric) delivery because it is soluble in water only at pH greater than about 7.0. The copolymer is easily extraded because it is flexible and because it has a low glass
transition temperature of approximately 48°C. The glass transition temperature is further reduced upon the addition of a plasticizer.
Anhydrous theophylline USP/NF is available from Spectrum Quality Products, Inc. (Gardena, CA). Polyethylene glycol 8000 (PEG 8000 or Carbowax® 8000) powder is available from Union Carbide (Danbury, CT). Microcrystalline cellulose (Avicel® PH- 101) is available from FMC (Newark, Delaware).
Referring to FIG. 1, the thermopolymer, active agent and one or more other pharmaceutical excipients are mixed prior to hot-melt extrasion to form a blended mass, generally a powder blend. After mixing, the blended mass is loaded into the hopper of the extrader and is extraded to form an extraded solid, or extradate. Depending upon the die employed for extrasion, an extradate with a predetermined shape will be formed. In the exemplary embodiment of FIG.l, a circular die is used. Therefore, the extradate is cylindrically shaped, i.e., it has a generally circular cross-section. The extradate is optionally cooled prior to being fed into a sizing apparatus, such as a pelletizer, where the extradate is then converted into cylindrical pellets or mini-tablets that are then spheronized while being heated in a spheronizer to form spherical particles.
Dies of different shapes and sizes can be used to form the extradate; however, cylindrical dies may provide more uniform particle size distribution and smoother surface of the resulting particles. Essentially any known extrasion die can be used. Depending upon the die used, the extradate will have a corresponding cross-sectional shape. Extrusion dies having geometrically-shaped or irregular cross-sections will generally be used. Accordingly, the extradate may be shaped as a rod, ribbon or other extradate conventionally formed in the plastics industry.
Cooling of the extradate prior to sizing is optional. Cooling can be conducted by exposing the extradate to cooler air, gas, or liquid. Cooling can be done be employing a cooling apparatus between the extruder and sizing apparatus or by exposing the extradate to a cooler ambience. The step of cooling generally renders the extradate more brittle and/or less pliable.
While a pelletizer is depicted in FIG. 1 as a sizing apparatus, other sizing equipment capable of reducing the extradate to plural smaller sized particles can be used.
The selection of the equipment may depend upon the extrasion die used and therefore the cross-sectional shape of the extradate. For example, an extrusion die with a round cross- section will form a cylindrical extradate, whereas, an extrusion die with a flat-lip will form a ribbon or sheet-shaped extradate. A pelletizer equipped with a cutting wheel or rolling knife will be useful for sizing the cylindrical extradate, whereas a sieve can be used for sizing the ribbon-shaped extradate by forcing the extradate through the sieve.
The particle size of the sized extradate generally ranges from 0.1 to 10 or 0.1 to 5 mm. Generally the ratio of the length to diameter of the sized particle ranges from about 0.1:10 to 10:0.1, 0.5:5.0 to 5.0-0.5, or about 1:1. Once the extradate has been converted into plural smaller sized particles, it is processed in a spheronizer to render the particles spherical. The spheronizer generally deforms the particles by a combination of mechanical forces and optional heat. When heat is applied to the mass being spheronized, the temperature will generally be at least 1-5 degrees above the Tg or melting point of the most abundant thermoformable material in the particles. For example, the spherical particles prepared according to Example 6A were heated to 170-235° F, which temperature is higher than the Tg of the plasticized Eudragit® 4135F polymer.
A first group of beads was made according to Example 1 by employing a conventional wet-mass granulation/spheronization process. A second group of beads was made according to Example 2 by employing the hot-melt extrasion/spheronization process of the invention. The polymer preparation Eudragit® 4135 F (Rohm; Darmstat, Germany) was employed as the formulation carrier, and anhydrous theophylline USP/NF (Spectrum Quality Products, Inc.; Gardena, CA) was used as the active agent. Carbowax® 8000 powder as supplied by Union Carbide (Polyethylene Glycol; Danbury, CT) was chosen as a plasticizing agent and lubricant to facilitate extrasion, and Avicel® PH-101 as supplied by FMC (Microcrystalline Cellulose; Newark, Delaware) was used as a wet-mass granulating aid in Example 1.
FIG. 2 A depicts a scanning electron micrograph (low magnification, 50 X) of the surface of a spherical particle produced by a conventional wet mass granulation spheronization process, and FIG. 3A depicts a cross-sectional scanning
electron micrograph (high magnification, 10,000X) of the interior of the same. Both the surface and the interior of the particle are rough, highly segregated (particulate) and not homogeneous. Distinct drag and excipient particle are visible in the wet-mass granulated bead. It is believed that this heterogeneous morphology of the matrix occurs because the polymer particles remain rigid during wet-mass granulation.
FIG. 2B depicts a scanning electron micrograph (low magnification, 50 X) of the surface of a spherical particle produced by the process of the invention. FIG. 3B depicts a cross-sectional scanning electron micrograph (high magnification, 10,000X) of the interior of the same. Both the surface and the interior of the particle are generally very smooth with very few isolated particles, except for an occasional dimple that might occur on the surface of the particle due to incomplete spheronization. Extending the spheronization time will eliminate the occurrence of the dimple. The isolated particles on the surface of the spheres are likely caused by the dusting of the particles during spheronization. In other words, the hot-melt extraded bead has a continuous matrix for the most part. Although the hot-melt extruded bead is very spherical, it does possess a dimple. Spheronization of hot-melt extruded pellets results in spheres with dimples on either side that was cut by the pelletizer. Increasing spheronization time, and optionally temperature, minimizes or eliminates the dimple. Other methods such as varying composition of the material to be hot-melt extruded may be used to minimize the dimple size or remove the dimple during spheronization.
FIG. 4 depicts the results of particle size distribution analyses on the beads of FIGS. 2 A and 2B. The mesh size number indicates the screen pore size; pore size decreases with increasing mesh size number. The most common particle size range for spherical pellets manufactured by both processes is mesh size numbers 16-14. Although both processes have a most common particle size range, hot-melt extrasion/spheronization produces many more beads in the size range. The beads produced by conventional wet- mass granulation/spheronization possess a wide particle size distribution. This wide distribution is generally a problem for formulations wherein additional coatings will be applied to the bead. On the other hand, the particle size distribution of beads produced by hot-melt extrusion/spheronization is substantially narrower. Approximately 50% or the wet-mass granulated/spheronized beads are of size 16-14, while 90% of the hot-melt
extruded/spheronized beads are size 16-14. Thus, hot-melt extrasion/spheronization is a more efficient process for producing spherical particles, beads, having a narrow particle size distribution. The invention thus provides a process for making a composition comprising spheronized particles having a narrow particle size distribution, wherein at least 75%, or at least 85% or at least 90% wt. of the particles are within +20% or within +10% of the desired average particle size.
Beads produced by the conventional wet-mass granulation/spheronization process and those produced by the process of the invention also differ substantially in their release of active agent. Although the composition and average particle size of the beads of FIGS. 2 A, 2B, 3 A and 3B are nearly identical, the difference in processing techniques results in beads with significantly different release profiles. The release profile of the beads was determined with a USP apparatus 2.
The release profiles of the wet-mass granulated/ spheronized and hot-melt extraded/spheronized beads were compared. Referring to FIGS. 5-8, the release profile for the spherical particles produced by wet-mass granulation/spheronization were identical regardless of the pH of the dissolution medium, as long as the pH was below the pH at which Eudragit® 4135 F is soluble (at least pH 6.5-7.0). In each case, the active agent theophylline was completely released from the wet-mass granulated sphere after approximately 4 hours. Although the drug was released quickly from these beads, the beads remained intact even after completion of the 12-hour dissolution test because the polymer is not significantly soluble in aqueous media having a pH less than 7.0. When the wet-mass granulated spheres were tested in a dissolution medium of pH 7.4, theophylline was completely released in approximately 2 hours. The theophylline release rate increased because the wet-mass granulated spheres disintegrated quickly, presenting no barrier to drag release.
On the other hand, the hot-melt extraded/spheronized beads exhibited more control over the release of theophylline. The theophylline release profiles in pH 1.2 (FIG. 5) and 3.0 (FIG. 6) are very similar, with only 52% of drug released after 12 hours. The hot-melt extruded spheres displayed an initial burst in drag release, due to theophylline on or near to the bead surface, but drug release was zero-order after the initial burst. It is believed that the release was zero-order because the polymer is insoluble at the evaluated
pH, and drug release is therefore limited by diffusion across the polymer barrier. When the hot-melt extruded spherical pellets were tested in a medium of pH 6.8 (FIG. 7), approximately 69% of the theophylline was released over 12 hours. It is believed that more theophylline was released at this higher pH because the pH of the dissolution medium (pH 6.8) was closer to the pH at which the polymer is starts to dissolve (>pH 7.0) and particles slowly began to swell at pH 6.8. Therefore, there would be less of a barrier to drug release, since the polymer is slightly soluble at pH 6.8. As noted above, the beads remained intact during dissolution testing at pH < 6.8. The theophylline release profile from hot-melt extruded spherical pellets in pH 7.4 is similar to that from wet-mass granulated spherical pellets in pH less than 7.4, with almost complete drag release attained after 4 hours of testing. However, unlike the wet-granulated beads that rapidly disintegrated at pH 7.4, the hot-melt extraded beads eroded slowly. Thus, the beads processed by hot-melt extrasion do not release theophylline as quickly as those processed by wet-mass granulation and also provide a greater control over the release of theophylline.
The dissolution tests conducted for FIGS. 5-8 demonstrate the behavior of the conventional beads and the beads of the invention at constant pH. The pH of the gastrointestinal tract of a human, however, changes from very acidic (stomach) to neutral or slightly basic (small intestines and colon). USP apparatus 3 is useful for mimicking the conditions to which the beads will be exposed in the GI tract. The Biodis™ apparatus can replicate transit through the human gastrointestinal track because it allows a single sample of beads to be subjected to multiple environments.
Figure 9 depicts the release profiles for the beads as they are exposed sequentially, in the order indicated, to solutions having pH values of 1.2, 3.0, 5.0, 6.8, and 7.4. Under these conditions, theophylline was completely released from the wet-mass granulated beads after 4 hours, which behavior is similar to that observed in the USP apparatus 2 at a pH of less than 7.4. Therefore, these conventional beads provided very little controlled release of theophylline. In contrast, the present inventors have discovered that the hot-melt extraded beads exhibit greater control over the release of drug. The initial burst in drug release was observed in pH 1.2, but drug release was zero-order through pH values 3.0, 5.0, and 6.8. At the 5-hour time point, which is one hour after
exposure to pH 6.8 has begun, only 35% of the theophylline was released. The remaining theophylline was released in the pH 7.4 medium. Accordingly, the invention also provides a method of converting a formulation from a pH independent one to a pH dependent one, the method comprising the steps of: hot-melt extruding a composition comprising at least one thermoformable material having a pH dependent solubility, at least one active agent and optionally a plasticizer to form an extraded solid, wherein the formulation provides a rapid or immediate release solid when prepared by wet-mass granulation/spheronization; and spheronizing the extraded solid in the presence of heat to form a pH dependent controlled or extended release hot-melt extraded/spheronized particle. Although the experimentally determined Tg of the hot-melt extraded/spheronized pellets was 23 °C, the pellets exhibited no sticking after storage for 1 week in sealed HPDE containers at 40°C/75% RH.
Formulations prepared by other methods and film coated with acrylate- methacrylate copolymers or ethylcellulose as retardant materials, are known to demonstrate unstable release profiles when the products are stored over extended periods. By unstable release profiles is meant that the release profile of a given formulation changes as storage time increases so that the release profile after an extended period of storage is significantly different than the initial release profile of the formulation. Theophylline release properties for hot-melt extraded/spheronized particles of the invention, however, did not change after storage for 1 year in sealed HDPE containers at 25°C/60% RH. For example, FIG. 10 depicts the release profiles for hot-melt extraded/spheronized beads prepared according to the invention initially (•) and after one year of storage (■). The release profiles are virtually identical meaning that the process of the invention, and the beads prepared thereby, can provide a stable release profile that does not change substantially over an extended period of storage.
The formulation of Example 7 is related to the formulation of Example 2, except that the formulation of Example 7 includes different amounts of the components that are in common, and the formulation of Example 7 includes magnesium stearate rather than microcrystalline cellulose. The release profiles for the formulation of Example 7 are depicted in FIG. 11, and the release profiles for the formulation of Example 2 are depicted in FIGS. 9 and 12. Both formulations exhibit a pH dependent release profile with the
formulation of Example 2 exhibiting a slightly faster release rate in lower pH media as compared to the formulation of Example 7 in the same media.
The formulation of Example 11 is related to the formulation of Example 2, except that the formulation of Example 11 includes diltiazem rather than theophylline as the active agent. The release profiles for the formulation of Example 11 are depicted in FIGS. 13 and 14. Both formulations exhibit a pH dependent release profile; however, the release rate for the formulation of Example 11 exhibits much less pH dependence than does the release profile of the formulation of Example 2 in the same media. The differences between the release profile properties of the two formulations can be attributed to the differences in the physical properties of the two active agents: theophylline is only soluble to about 0.0083 g/ml of water, whereas diltiazem hydrochloride is freely soluble in water.
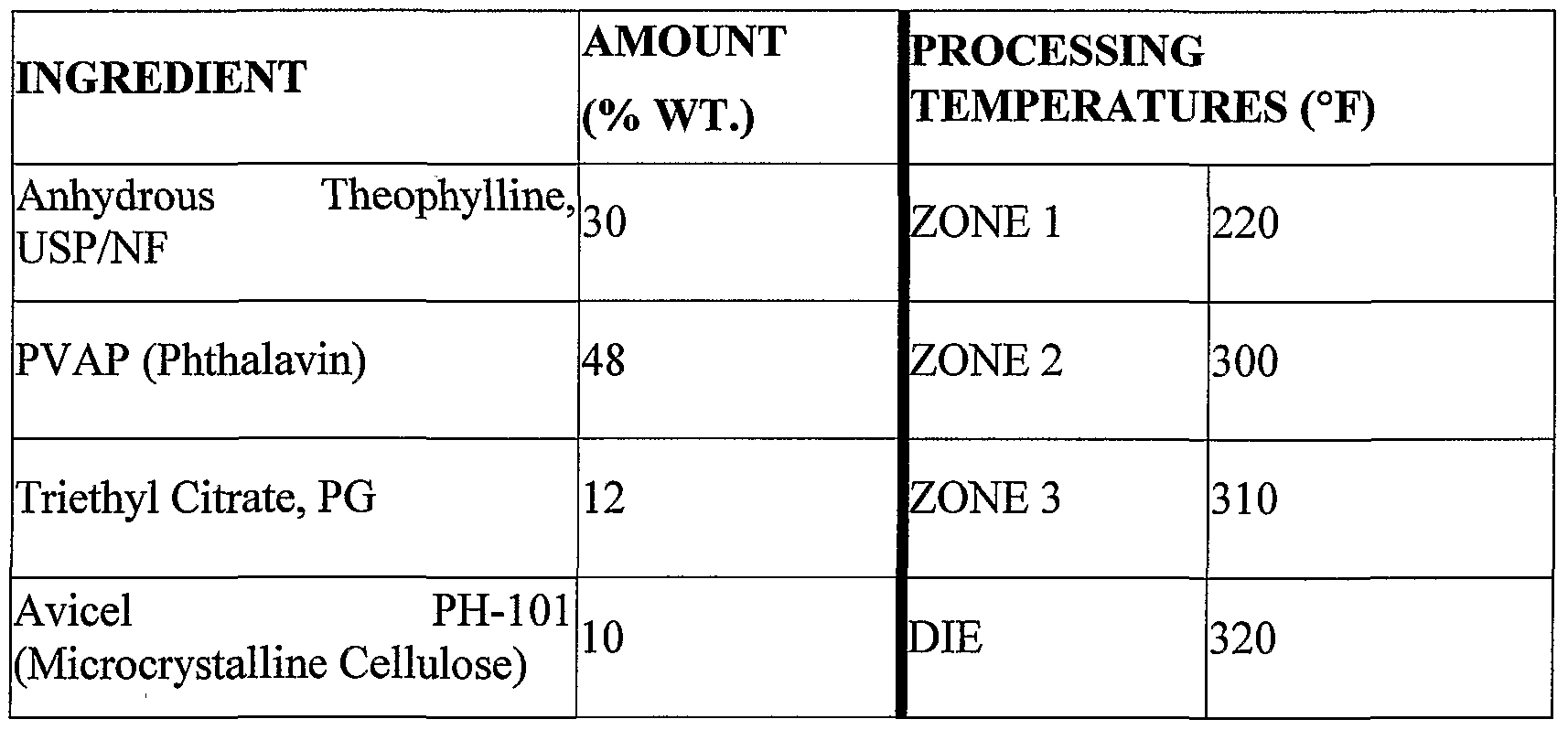
The formulation of Example 12 is only somewhat related to the formulation of Example 2. The formulation of Example 12 includes the polymer EUDRAGIT™ RS PO rather than EUDRAGIT™ 4135F and includes sorbitol rather than microcrystalline cellulose. EUDRAGIT™ RS PO (Rohm America; See USP 24/NF 19 page 2413) is a pH independent polymer with low permeability generally intended for use in matrix formulations. However, EUDRAGIT™ RS PO differs from EUDRAGIT™ 4135F in that the latter polymer starts to dissolve in aqueous media at pH 7.0 and the Tg of the RS PO polymer is about 55-56°C. The release profiles for the formulation of Example 12 are depicted in FIG. 15. Although they share some common properties, the EUDRAGIT™ RS PO formulation exhibits a lower degree of pH dependence in its release profile.
The formulation of Example 13 is only somewhat related to the formulation of Example 2. The formulation of Example 13 includes a combination of the polymers EUDRAGIT™ RS PO and EUDRAGIT™ S100 rather than EUDRAGIT™ 4135F and includes talc rather than microcrystalline cellulose. Also, the components they have in common are present in different amounts. EUDRAGIT™ S100 (Rohm America; See USP 24/NF 19 page 2477) is a pH dependent anionic polymer that containing carboxyl groups that ionize at about pH 7.0 and above thereby allowing the polymer to pass into solution. It is generally used for formulations that provide a targeted drag delivery in the colon. EUDRAGIT™ preparation 4135F is stracturally similar to EUDRAGIT™ S100 but has a lower Tg and is less brittle and more plastic than EUDRAGIT™ S100. The release
profiles for the formulation of Example 13 are depicted in FIG. 16. The formulation of Example 13 exhibits a lower degree of pH dependence in its release profile even though it comprises a significant amount of the pH dependent polymer EUDRAGIT™ SI 00.
The porosity of pellets manufactured by both wet-mass extrusion/spheronization and hot-melt extrusion/spheronization was determined because the matrix-controlled diffusion theory assumes that drug diffuses though intergranular pores in the matrix system. A porous dosage form should exhibit a faster drag release rate since it has more pores or channels for water to enter and to dissolve drag. The porosity measurements were calculated using data from helium pycnometry and mercury porosymetry experiments. Porosities of the wet-mass extruded and of the hot-melt extruded spherical pellets were significantly different (n = 3, p < 0.05), as illustrated by the cross-sectional SEM pictures of FIGS. 3A and 3B. The porosity of the wet-mass extruded beads was 6.09 ± 0.08, whereas the porosity of the hot-melt extraded beads was 3.70 ± 0.08. The hot- melt-extruded beads were less porous due to a decrease free volume of the polymer matrix. The elevated temperatures and high pressures utilized in hot-melt extrusion reduced the free volume of the resulting exudates. The polymer chain interactions of the molten polymers are increased under these elevated conditions.
Accordingly, the combination process of the invention possesses several advantages over the conventional wet-mass granulation/spheronization process of making beads. The hot-melt extrusion/spheronization process can be performed semi- continuously or continuously because it does not require a lengthy drying step and it does not require the addition of water or other solvents. The hot melt-extrasion/spheronization process produces spherical beads having a narrower particle size distribution than does the conventional wet-mass granulation/spheronization process. When an identical dry powder formulation was employed, the hot-melt extruded beads exhibited greater control over drug release than the wet-mass granulated beads. The beads produced by hot-melt extrasion/spheronization possess a more homogeneous internal matrix and surface than do the beads produced by wet-mass granulation/spheronization. The beads produced by hot- melt extrasion spheronization provide a greater control over the release of active agent than do the beads produced by wet-mass granulation/spheronization. The hot-melt extrusion/spheronization process produces much less waste than the wet-mass
granulation/spheronization process because the former process produces a greater percentage of beads having the desired particle size and does not employ solvents.
As used herein, the glass transition temperature is taken to mean the temperature at which a solid material softens or melts and passes from a glassy to a more rubbery state. The process of the present invention can be used on any thermoformable material suitable for hot-melt extrasion. Generally, a suitable thermoformable material will have a melting point, glass transition temperature or softening temperature of less than the decomposition temperatures of components in the composition before or at least after the addition of a plasticizer. Some factors that may affect the temperature at which the composition being extraded is extraded: 1) the melting or softening temperature of the thermoformable material as well as other materials in the composition; 2) the back-pressure created in the extrader; 3) the die size; 4) the viscosity of the composition during extrasion; 5) the speed of the screws during extrasion; and/or 6) the physical properties of the components of the composition.
Suitable thermoformable materials that may or may not require a plasticizer include, for example, Eudragit™ RS PO, Eudragit™ SI 00, Kollidon SR (poly(vinyl acetate)-co-poly(vinylpyrrolidone) copolymer), Ethocel™ (ethylcellulose), HPC (hydroxypropylcellulose), cellulose acetate butyrate, poly(vinylpyrrolidone) (PVP), poly(ethylene glycol) (PEG), poly(ethylene oxide) (PEO), poly(vinyl alcohol) (PVA), hydroxypropyl methylcellulose (HPMC), ethylcellulose (EC), hydroxyethylcellulose (HEC), sodium carboxymethyl-cellulose (CMC), dimethylaminoethyl methacrylate - methacrylic acid ester copolymer, ethylacrylate - methylmethacrylate copolymer (GA-MMA), C-5 or 60 SH-50 (Shin-Etsu Chemical Corp.), cellulose acetate phthalate (CAP), cellulose acetate trimelletate (CAT), poly(vinyl acetate) phthalate (PVAP), hydroxypropylmethylcellulose phthalate (HPMCP), poly(methacrylate ethylacrylate) (1:1) copolymer (MA-EA), poly(methacrylate methylmethacrylate) (1:1) copolymer (MA-MMA), poly(methacrylate methylmethacrylate) (1:2) copolymer, spray-dried Eudragit L-30-D™ (MA-EA, 1:1), Eudragit L-100-55™ (MA-EA, 1:1), hydroxypropylmethylcellulose acetate succinate (HPMCAS), Coateric™ (PVAP), Aquateric™ (CAP), AQUACOAT™ (HPMCAS), or combinations thereof.
Other suitable materials include, for example, cellulose acylate, cellulose diacylate, cellulose triacylate, cellulose acetate, cellulose diacetate, cellulose triacetate; mono, di and tricellulose alkanylates; mono, di and tricellulose aroylates; cellulose acetate having a D.S. up to 1 and an acetyl content up to 21%; cellulose acetate having an acetyl content of 32 to 39.8%; cellulose diacetate having a D.S. of 1 to 2 and an acetyl content of 21 to 35%; cellulose triacetate having a D.S. of 2 to 3 and an acetyl content of 35 to 44.8%; cellulosic polymers include cellulose propionate having a D.S. of 1.8 and a propionyl content of 39.2 to 45% and a hydroxyl content of 2.8 to 5.4%; cellulose acetate butyrate having a D.S. of 1.8, an acetyl content of 13 to 15% and a butyryl content of 34 to 39%; cellulose acetate butyrate having an acetyl content of 2 to 29%; a butyryl content of 17 to 53% and a hydroxyl content of 0.5 to 4.7%; cellulose triacylates having a D.S. of 2.9 to 3 such as cellulose trivalerate, cellulose trilaurate, cellulose tripalmitate, cellulose trisuccinate, and cellulose trioclanoate; cellulose diacylates having a D.S. of 2.2 to 2.6 such as cellulose disuccinate, cellulose dipalmitate, cellulose dioclanoate, cellulose dipentale; acetaldehyde dimethyl acetate, cellulose acetate ethyl carbamate, cellulose acetate methyl carbamate, cellulose acetate dimethyl aminoacetate, polyamides, polyurethanes, sulfonated polystyrenes, cross-linked polymers formed by the coprecipitation of a polyanion and a polycation as disclosed in U.S. Patents No. 3,173,876, No. 3,276,586, No. 3,541,005, No. 3,541,006, and No. 3,546,142; polymers as disclosed by Loeb and Sourirajan in U.S. Pat. No. 3,133,132; lightly cross-linked polystyrene derivatives; cross-linked poly(sodium styrene sulfonate), cross-linked poly(vinylbenzyltrimethyl ammonium chloride); other polymers as disclosed in U.S. Patents No. 3,845,770, No. 3,916,899, No. 4,765,989 and No. 4,160,020 and in Handbook of Common Polymers (Scott, J. R. and Roff, W. J., eds.; 1971; CRC Press, Cleveland, Ohio). Still other suitable materials include, for example, keratin, keratin sandarac-tolu, salol (phenyl salicylate), salol beta-naphthylbenzoate and acetotannin, salol with balsam of Peru, salol with tolu, salol with gum mastic, salol and stearic acid, and salol and shellac; a member selected from the group consisting of formalized protein, formalized gelatin, and formalized cross-linked gelatin and exchange resins; a member selected from the group consisting of myristic acid-hydrogenated castor oil-cholesterol, stearic acid-mutton tallow, stearic acid-balsam of tolu, and stearic acid-castor oil; a member selected from the group consisting of shellac, ammoniated shellac, ammoniated shellac-salol, shellac-wool fat,
shellac-acetyl alcohol, shellac-stearic acid-balsam of tolu, and shellac n-butyl stearate; a member selected from the group consisting of abietic acid, methyl abictate, benzoin, balsam of tolu, sandarac, mastic with tolu, and mastic with tolu, and mastic with acetyl alcohol; acrylic resins represented by anionic polymers synthesized from methacrylate acid and methacrylic acid methyl ester, copolymeric acrylic resins of methacrylic and methacrylic acid and methacrylic acid alkyl esters, copolymers of alkacrylic acid and alkacrylic acid alkyl esters, acrylic resins such as dimethylaminoethylmethacrylate- butylmethacrylate-methylmethacrylate copolymer of 150,000 molecular weight, methacrylic acid-methyl methacrylate 50:50 copolymer of 135,000 molecular weight, methacrylic acid-methylmethacrylate-30:70-copolymer of 135,000 mol. wt., methacrylic acid-dimethylaminoethyl methacrylate-ethyl acrylate of 750,000 mol. wt., methacrylic acid-methyl methacrylate-ethyl acrylate of 1,000,000 mol. wt., and ethyl acrylate-methyl methacrylate-ethyl acrylate of 550,000 mol. wt; an enteric composition comprising a member selected from the group consisting of cellulose acetyl phthalate, cellulose diacetyl phthalate, cellulose triacetyl phthalate, sodium cellulose acetate phthalate, cellulose ester phthalate, cellulose ether phthalate, methylcellulose phthalate, cellulose ester-ether phthalate, hydroxypropyl cellulose phthalate, alkali salts of cellulose acetate phthalate, alkaline earth salts of cellulose acetate phthalate, calcium salt of cellulose acetate phthalate, ammonium salt of hydroxypropyl methylcellulose phthalate, cellulose acetate hexahydrophthalate, hydroxypropyl methylcellulose hexahydrophthalate, polyvinyl acetate phthalate diethyl phthalate, dibutyl phthalate, dialkyl phthalate wherein the alkyl comprises from 1 to 7 straight and branched alkyl groups, aryl phthalates; water soluble polysaccharide gums such as carrageenan, fucoidan, gum ghatti, tragacanth, arabinogalactan, pectin, and xanthan; water-soluble salts of polysaccharide gums such as sodium alginate, sodium tragacanthin, and sodium gum ghattate; a blend of gelatin and polyvinyl-pyrrolidone; gelatin; water-soluble hydroxyalkylcellulose wherein the alkyl member is straight or branched of 1 to 7 carbons such as hydroxymethylcellulose; synthetic water-soluble cellulose-based lamina formers such as methyl cellulose and its hydroxyalkyl methylcellulose; cellulose derivatives such as a member selected from the group consisting of hydroxyethyl methylcellulose and hydroxybutyl methylcellulose; croscarmellose sodium; copovidone; poly(ethylene-vinyl acetate) (60:40) copolymer (EVAC from Aldrich Chemical Co.), 2-hydroxyethylmethacrylate (HEMA), MMA,
terpolymers of HEMA:MMA:MA synthesized in the presence of N,N'- bis(methacryloyloxyethyloxycarbonylamino)-azobenzene, azopolymers, enteric coat timed release system (Time Clock® from Pharmaceutical Profiles, Ltd., UK); and calcium pectinate. All of the above suitable materials can be used alone or in various independent combinations.
Other suitable materials that can be used as the thermoformable material include wax, protein, cellulosic polymer, polyol, nonelectrolyte, acrylic polymer, fat, glycerin, lipid, fatty acid, fatty alcohol, carbomer, polyvinyl polymer, or a combination thereof. Specific examples include carnauba wax, bees wax, monoglyceride, diglyceride, triglyceride, polysaccharide, HPC, HPMC, poly(ethylene oxide), ethyl cellulose, (carbopol 97 IP Polycarbopbil), HPMCAS, HPMCP, polyvinyl acetate, cellulose acetate, cellulose acetate butyrate, polyethylene polymer, or combinations thereof. These materials may be further described as carriers, and may be used alone or in combination with other materials of the present disclosure. As used herein, a cellulosic polymer is a polymer or copolymer that is based upon cellulose and includes derivatives thereof. As used herein, a polyvinyl or polyethylene polymer is a polymer or copolymer based upon ethylene or a derivative of ethylene. As used herein, a polyol is a polyhydroxylated compound or polymer and includes, by way of example and without limitation, mannitol, xylitol, and sorbitol. As used herein, an acrylic polymer is a polymer or copolymer that is based upon acrylic acid, an acrylate ester, an alkacrylic acid, and/or an alkacrylic ester and includes derivatives thereof. Exemplary monomers for use in an acrylic polymer include acrylic acid, methacrylic acid, alkyl methacrylate, alkyl acrylate, aromatic methacrylate, amino functionalized methacrylate, quaternary functionalized methacrylate, acid functionalized methacrylate, ether functionalized methacrylate, triethylene glycol monoethylether methacrylate, tefrahydrofurfuryl-2-methacrylate, N, N-dimethylaminoethyl methacrylate, N-dimethylaminopropyl methacrylamide, 2-tert-butylaminoethyl methacrylate; 2-frimemylammoniummethyl methacrylate chloride, methacrylamidopropyltrimethyl- ammonium chloride, methacrylamide, N-methylol methacrylamide, acrylamide, ethyl methacrylate, butyl methacrylate, isodecyl methacrylate, cyclohexyl methacrylate, 2-ethylhexyl methacrylate, isobornyl methacrylate, benzyl methacrylate, 3,3,5-
trimethylcyclohexyl methacrylate, lauryl methacrylate, stearyl methacrylate, 2- hydroxyethyl acrylate, hydroxypropyl acrylate, 2-hydroxyethyl methacrylate, hydroxypropyl methacrylate, glycerolmonomethacrylate, and combinations thereof.
Some of the materials listed above may be too brittle or may have Tg values that are generally too high rendering them too difficult to extrude. Such materials can be combined with one or more plasticizers to render them thermoformable. As used herein, a plasticizer is a first material that reduces the melting point, softening temperature or Tg of a second material. As a consequence, a second material that is not thermoformable can be made thermoformable by the addition of a plasticizer. Plasticizers, such as low molecular weight PEG, generally broaden the average molecular weight of a polymer in which they are included thereby lowering its glass transition temperature or softening point. Plasticizers also generally reduce the viscosity of a polymer. It is possible the plasticizer will impart some particularly advantageous physical properties to the osmotic device of the invention.
Plasticizers useful in the invention can include, by way of example and without limitation, low molecular weight polymers, oligomers, copolymers, oils, small organic molecules, low molecular weight polyols having aliphatic hydroxyls, ester-type plasticizers, glycol ethers, poly(propylene glycol), multi-block polymers, single block polymers, low molecular weight poly(ethylene glycol), citrate ester-type plasticizers, triacetin, propylene glycol and glycerin. Such plasticizers can also include ethylene glycol, 1,2-butylene glycol, 2,3-butylene glycol, styrene glycol, diethylene glycol, triethylene glycol, tetraethylene glycol and other poly(ethylene glycol) compounds, monopropylene glycol monoisopropyl ether, propylene glycol monoethyl ether, ethylene glycol monoethyl ether, diethylene glycol monoethyl ether, sorbitol lactate, ethyl lactate, butyl lactate, ethyl glycolate, dibutylsebacate, acetyltributylcitrate, triethyl citrate, acetyl triethyl citrate, tributyl citrate and allyl glycolate. All such plasticizers are commercially available from sources such as Aldrich or Sigma Chemical Co. It is also contemplated and within the scope of the invention, that a combination of plasticizers may be used in the present formulation. The PEG based plasticizers are available commercially or can be made by a variety of methods, such as disclosed in Poly(ethylene glycol) Chemistry: Biotechnical and Biomedical Applications (J.M. Harris, Ed.; Plenum Press, NY) the disclosure of which is hereby incorporated by reference.
The composition that is to be hot-melt extraded can further comprise other pharmaceutical excipients including, for example, release-modifying agents, bulking agents, processing agents, antioxidant, acidifying agent, alkalizing agent, buffering agent, preservative, adsorbent, sweetening agent, antiadherent, binder, lubricant, diluent, direct compression excipient, glidant, lubricant, opaquant, polishing agent, disintegrant, flavorant, colorant, osmotic agent, release-modifying agents, bulking agents, and processing agents.
As used herein, the term "antioxidant" is intended to mean an agent that inhibits oxidation and thus is used to prevent the deterioration of preparations by oxidation. Such compounds include, by way of example and without limitation, ascorbic acid, ascorbyl palmitate, butylated hydroxyanisole, butylated hydroxytoluene, hypophophorous acid, monothiogfycerol, sodium ascorbate, sodium formaldehyde sulfoxylate and sodium metabisulfite and others known to those of ordinary skill in the art.. Other suitable antioxidants include, for example, vitamin C, BHT, BHA, sodium bisulfite, vitamin E and its derivatives, propyl gallate or a sulfϊte derivative.
A buffering agent is used to resist change in pH upon dilution or addition of acid or alkali. Such compounds include, by way of example and without limitation, potassium metaphosphate, potassium phosphate, monobasic sodium acetate and sodium citrate anhydrous and dihydrate, salts of inorganic or organic acids, salts of inorganic or organic bases, and others known to those of ordinary skill in the art.
As used herein, the term "acidifying agent" is intended to mean a compound used to provide an acidic medium for product stability. Such compounds include, by way of example and without limitation, acetic acid, amino acid, citric acid, fumaric acid and other alpha hydroxy acids, such as hydrochloric acid, ascorbic acid, and nitric acid and others known to those of ordinary skill in the art.
As used herein, the term "alkalizing agent" is intended to mean a compound used to provide alkaline medium for product stability. Such compounds include, by way of example and without limitation, ammonia solution, ammonium carbonate, diethanolamine, monoethanolamine, potassium hydroxide, sodium borate, sodium carbonate, sodium bicarbonate, sodium hydroxide, triethanolamine, and trolamine and others known to those of ordinary skill in the art.
Preservatives include compounds used to prevent the growth of microorganisms.
Suitable preservatives include, by way of example and without limitation, benzalkonium chloride, benzethonium chloride, benzyl alcohol, cetylpyridinium chloride, chlorobutanol, phenol, phenylethyl alcohol, phenylmercuric nitrate and thimerosal and others known to those of ordinary skill in the art.
As used herein, the term "adsorbent" is intended to mean an agent capable of holding other molecules onto its surface by physical or chemical (chemisorption) means. Such compounds include, by way of example and without limitation, powdered and activated charcoal and other materials known to one of ordinary skill in the art. As used herein, the term "sweetening agent" is intended to mean a compound used to impart sweetness to a preparation. A sweetening agent may be included in the time-release coating or other exterior coating of the tablet. Such compounds include, by way of example and without limitation, aspartame, dextrose, glycerin, mannitol, saccharin sodium, sorbitol and sucrose and other materials known to one of ordinary skill in the art. As used herein, the term "antiadherent" is intended to mean an agent that prevents the sticking of solid formulation ingredients to punches and dies in a machine, for example, during production. Such compounds include, by way of example and without limitation, magnesium stearate, talc, calcium stearate, glyceryl behenate, PEG, hydrogenated vegetable oil, mineral oil, stearic acid and other materials known to one of ordinary skill in the art.
As used herein, the term "binder" is intended to mean substances generally used to cause adhesion of powder particles in solid granulations. These materials may include the above-listed thermoformable materials. Moreover, such compounds may include, by way of example and without limitation, acacia, alginic acid, carboxymethylcellulose sodium, poly(vinylpyrrolidone), compressible sugar (e.g., NuTab), ethylcellulose, gelatin, liquid glucose, methylcellulose, povidone and pregelatinized starch and other materials known to one of ordinary skill in the art. Other exemplary binders include acacia, tragacanth, gelatin, starch, cellulose materials such as methyl cellulose and sodium carboxy methyl cellulose, . alginic acids and salts thereof, polyethylene glycol, guar gum, polysaccharide, bentonites, sugars, invert sugars, poloxamers (PLURONIC™ F68, PLURONIC™ F127), collagen, albumin, gelatin, cellulosics in nonaqueous solvents,
combinations thereof and the like. Other binders include, for example, polypropylene glycol, polyoxyethylene-polypropylene copolymer, polyethylene ester, polyethylene sorbitan ester, polyethylene oxide, combinations thereof and other materials known to one of ordinary skill in the art. As used herein, the term "diluent" or "filler" is intended to mean inert substances used as fillers to create the desired bulk, flow properties, and compression characteristics in the preparation of the cores. Such compounds include, by way of example and without limitation, dibasic calcium phosphate, kaolin, lactose, sucrose, mannitol, microcrystalline cellulose (Avicel™ PH-101), powdered cellulose, precipitated calcium carbonate, sorbitol, and starch and other materials known to one of ordinary skill in the art.
As used herein, the term "direct compression excipient" is intended to mean a compound used in direct compression formulations. Such compounds include, by way of example and without limitation, dibasic calcium phosphate (e.g., Ditab) and other materials known to one of ordinary skill in the art. As used herein, the term "dusting" is intended to mean the step of contacting a powdered pharmaceutical excipient with the solid extradate or sized particle either prior to or during spheronization. Generally, the dusting material that adheres to the surface of the spheronized particle is less than 5% wt. of the total bead weight.
As used herein,- the term "glidant" is intended to mean agents generally used in tablet or capsule formulations to reduce friction during tablet compression. Such compounds include, by way of example and without limitation, colloidal silica, cornstarch, fumed silica (Cab-O-Sil™) talc (Alphafil™ 500 USP), calcium silicate, magnesium silicate, colloidal silicon, silicon hydrogel and other materials known to one of ordinary skill in the art. As used herein, the term "lubricant" is intended to mean substances generally used in tablet formulations to reduce friction during tablet compression. Such compounds include, by way of example and without limitation, calcium stearate, magnesium stearate, mineral oil, stearic acid, and zinc stearate and other materials known to one of ordinary skill in the art.
As used herein, the term "opaquant" is intended to mean a compound used to render a coating opaque. May be used alone or in combination with a colorant. Such compounds include, by way of example and without limitation, titanium dioxide and other materials known to one of ordinary skill in the art. As used herein, the term "polishing agent" is intended to mean a compound used to impart an attractive sheen to coated cores. Such compounds include, by way of example and without limitation, carnauba wax, white wax and other materials known to one of ordinary skill in the art.
As used herein, the term "disintegrant" is intended to mean a compound used in solid dosage forms to promote the disraption of the solid mass into smaller particles which are more readily dispersed or dissolved. Exemplary disintegrants include, by way of example and without limitation, starches such as corn starch, potato starch, pre-gelatinized and modified starches thereof, sweeteners, clays, such as bentonite, microcrystalline cellulose(e.g., Avicel), carboxymethylcellulose calcium, cellulose polyacrilin potassium (e.g., Amberlite), alginates, sodium starch glycolate, gums such as agar, guar, locust bean, karaya, pectin, tragacanth and other materials known to one of ordinary skill in the art.
As used herein, the term "colorant" is intended to mean a compound used to impart color to solid (e.g., tablets) pharmaceutical preparations. Such compounds include, by way of example and without limitation, FD&C Red No. 3, FD&C Red No. 20, FD&C Yellow No. 6, FD&C Blue No. 2, D&C Green No. 5, D&C Orange No. 5, D&C Red No. 8, caramel, and ferric oxide, red, other F.D. & C. dyes and natural coloring agents such as grape skin extract, beet red powder, beta-carotene, annato, carmine, turmeric, paprika, and other materials known to one of ordinary skill in the art. The amount of coloring agent used will vary as desired. As used herein, the term "flavorant" is intended to mean a compound used to impart a pleasant flavor and often odor to a pharmaceutical preparation. Exemplary flavoring agents or flavorants include synthetic flavor oils and flavoring aromatics and/or natural oils, extracts from plants, leaves, flowers, fruits and so forth and combinations thereof. These may also include cinnamon oil, oil of wintergreen, peppermint oils, clove oil,, bay oil, anise oil, eucalyptus, thyme oil, cedar leave oil, oil of nutmeg, oil of sage, oil of bitter almonds and cassia oil. Other useful flavors include vanilla, citrus oil, including
lemon, orange, grape, lime and grapefruit, and fruit essences, including apple, pear, peach, strawberry, raspberry, cherry, plum, pineapple, apricot and so forth. Flavors that have been found to be particularly useful include commercially available orange, grape, cherry and bubble gum flavors and mixtures thereof. The amount of flavoring may depend on a number of factors, including the organoleptic effect desired. Flavors will be present in any amount as desired by those of ordinary skill in the art. Particularly preferred flavors are the grape and cherry flavors and citrus flavors such as orange.
Exemplary osmagents or osmotic agents include organic and inorganic compounds such as salts, acids, bases, chelating agents, sodium chloride, lithium chloride, magnesium chloride, magnesium sulfate, lithium sulfate, potassium chloride, sodium sulfite, calcium bicarbonate, sodium sulfate, calcium sulfate, calcium lactate, d-mannitol, urea, tartaric acid, raffinose, sucrose, alpha-d-lactose monohydrate, glucose, combinations thereof and other similar or equivalent materials which are widely known in the art.
The dosage form of the invention can also include oils, for example, fixed oils, such as peanut oil, sesame oil, cottonseed oil, corn oil and olive oil; fatty acids, such as oleic acid, stearic acid and isotearic acid; and fatty acid esters, such as ethyl oleate, isopropyl myristate, fatty acid glycerides and acetylated fatty acid glycerides. It can also be mixed with alcohols, such as ethanol, isopropanol, hexadecyl alcohol, glycerol and propylene glycol; with glycerol ketals, such as 2,2-dimethyl-l,3-dioxolane-4-methanol; with ethers, such as poly(ethyleneglycol) 450, with petroleum hydrocarbons, such as mineral oil and petrolatum; with water, or with mixtures thereof; with or without the addition of a pharmaceutically suitable surfactant, suspending agent or emulsifying agent.
Soaps and synthetic detergents may be employed as surfactants and as vehicles for detergent compositions. Suitable soaps include fatty acid alkali metal, ammonium, and triethanolamine salts. Suitable detergents include cationic detergents, for example, dimethyl dialkyl ammonium halides, alkyl pyridinium halides, and alkylamine acetates; anionic detergents, for example, alkyl, aryl and olefin sulfonates, alkyl, olefin, ether and monoglyceride sulfates, and sulfosuccinates; nonionic detergents, for example, fatty amine oxides, fatty acid alkanolamides, and poly(oxyethylene)-b/oc£-poly(oxypropylene) copolymers; and amphoteric detergents, for example, alkyl β-aminopropionates and 2- alkylimidazoline quaternary ammonium salts; and mixtures thereof.
Various other components, not otherwise listed above, can be added to the present formulation including, by way of example and without limitation, glycerylmonostearate (Imwittor™ 900), nylon, cellulose acetate butyrate, d,l-poly(lactic acid), 1,6 - hexanediamine, diethylenetriamine, starches, derivatized starches, acetylated monoglycerides, gelatin coacervates, poly (styrene - maleic acid) copolymer, glycowax, castor wax, stearyl alcohol, glycerol palmitostearate, poly(ethylene), poly(vinyl acetate), poly(vinyl chloride), 1,3 - butylene-glycoldimethacrylate, ethyleneglycol-dimethacrylate and methacrylate hydrogels.
It should be understood, that compounds used in the art of pharmaceutical formulation generally serve a variety of functions or purposes. Thus, if a compound named herein is mentioned only once or is used to define more than one term herein, its purpose or function should not be construed as being limited solely to that named purpose(s) or function(s).
The beads of the invention will together comprise an effective amount of an active agent when included in a dosage form. By the term "effective amount", it is understood that, with respect to, for example, pharmaceuticals, a therapeutically effective amount is contemplated. A therapeutically effective amount is the amount or quantity of drag that is sufficient to elicit the required or desired therapeutic response, or in other words, the amount that is sufficient to elicit an appreciable biological response when administered to a patient.
As used herein, "pharmaceutically acceptable salts" refer to derivatives of the disclosed compounds wherein the therapeutic compound is modified by making acid or base salts thereof. Examples of pharmaceutically acceptable salts include, but are not limited to, mineral or organic acid salts of the drug. The pharmaceutically acceptable salts include the conventional non-toxic salts, for example, from non-toxic inorganic or organic acids. For example, such conventional non-toxic salts include those derived from inorganic acids such as hydrochloric, hydrobromic, sulfuric, sulfonic, sulfamic, phosphoric, nitric and the like; and the salts prepared from organic acids such as amino acids, acetic, propionic, succinic, glycolic, stearic, lactic, malic, tartaric, citric, ascorbic, pamoic, maleic, hydroxymaleic, phenylacetic, glutamic, benzoic, salicylic, sulfanilic, 2- acetoxybenzoic, fumaric, toluenesulfonic, methanesulfonic, ethane disulfonic, oxalic,
isethionic, and other known to those of ordinary skill in the pharmaceutical sciences. Lists of suitable salts are found in texts such as Remington's Pharmaceutical Sciences, 18th Ed. (Alfonso R. Gennaro, ed.; Mack Publishing Company, Easton, PA, 1990); Remington: the Science and Practice of Pharmacy 19 Ed.( Lippincott, Williams & Wilkins, 1995); Handbook of Pharmaceutical Excipients, 3rd Ed. (Arthur H. Kibbe, ed.; Amer. Pharmaceutical Assoc, 1999); the Pharmaceutical Codex: Principles and Practice of Pharmaceutics 12th Ed. (Walter Lund ed.; Pharmaceutical Press, London, 1994); The United States Pharmacopeia: The National Formulary (United States Pharmacopeial Convention); and Goodman and Gilman 's: the Pharmacological Basis of Therapeutics (Louis S. Goodman and Lee E. Limbird, eds.; McGraw Hill, 1992), the disclosures of which are hereby incorporated by reference.
The phrase "pharmaceutically acceptable" is employed herein to refer to those compounds, materials, compositions, and/or dosage forms which are, within the scope of sound medical judgment, suitable for use in contact with the tissues of human beings and animals without excessive toxicity, irritation, allergic response, or other problem or complication, commensurate with a reasonable benefit/risk ratio.
The process of the invention can be used to prepare controlled release beads comprising essentially any one or more active agents. Active agents include physiological substances or pharmacological active substances that produce a systemic or localized effect or effects on animals and human beings. Active agents also include pesticides, herbicides, insecticides, antioxidants, plant growth instigators, sterilization agents, catalysts, chemical reagents, food products, nutrients, cosmetics, vitamins, minerals, dietary supplements, sterility inhibitors, fertility instigators, microorganisms, flavoring agents, sweeteners, cleansing agents and other such compounds for pharmaceutical, veterinary, horticultural, household, food, culinary, agricultural, cosmetic, industrial, cleansing, confectionery and flavoring applications. The active agent can be present in its neutral, ionic, salt, basic, acidic, natural, synthetic, diastereomeric, isomeric, enantiomerically pure, racemic, hydrate, chelate, derivative, analog, or other common form. Further therapeutic compounds which can be formulated into the present osmotic devices also include antibacterial substances, antiMstamines and decongestants, anti-
inflammatories, antiparasitics, antivirals, local anesthetics, antifungal, amoebicidal, or trichomonocidal agents, analgesics, antiarthritics, antiasthmatics, anticoagulants, anticonvulsants, antidepressants, antidiabetics, antineoplastics, antipsychotics, neuroleptics, antihypertensives, muscle relaxants, depressants, hypnotics, sedatives, psychic energizers, tranquilizers, anti-convulsants, antiparkinson agents, muscle contractants, anti-microbials, antimalarials, hormonal agents, contraceptives, sympathomimetics, diuretics, hypoglycemics, ophthalmics, electrolytes, diagnostics agents, cardiovascular drags, other types of therapeutic compounds known to those of ordinary skill in the pharmaceutical sciences, and combinations thereof. After the hot-melt extraded/spheronized beads are prepared they can be incorporated into a pharmaceutical dosage form by: 1) compressing the beads, with or without an additional pharmaceutical excipient, to form a tablet or tablet core, which is optionally subsequently coated; 2) loading the beads into a capsule; 3) coating the beads with one or more types of materials disclosed herein or generally used in the pharmaceutical sciences to prepare solid dosage forms (See Example 8) to further control release of the drag or provide a loading dose of drag; or 4) any combination of the above. Alternatively, the beads can be administered as is, i.e. loose or enclosed in a pouch or sachet. One or more coatings can be applied to the particles of the invention and/or to dosage forms containing the particles of the invention. The one or more coatings are independently selected at each occurrence from the group consisting of a release rate modifying coating, an enteric release coating, a rapid release coating, a colonic release coating, a delayed release coating, an immediate release coating, a taste-masking coating and a combination thereof.
Suitable solid dosage forms into which the hot-melt extraded/spheronized particles of the invention can be included include a pill, tablet, capsule, suspension, osmotic device, bead, granule, spheroid, particulate, paste, reconstitutable solid, pastille, pill, gelcap, troche, stick, suppository, implant, lollipop, patch, candy, food product or topical formulation.
In view of the above description and the examples below, one of ordinary skill in the art will be able to practice the invention as claimed without undue experimentation.
The foregoing will be better understood with reference to the following examples that
detail certain procedures for the preparation of formulations according to the present invention. All references made to these examples are for the purposes of illustration. The following examples should not be considered exhaustive, but merely illustrative of only a few of the many embodiments contemplated by the present invention.
EXAMPLE 1
The following process was used to make beads according to a conventional wet- mass granulation/spheronization process. The following ingredients in the amounts indicated were used.
The Eudragit® 4135 F was first ground since it is generally supplied as large granules. All powders were passed through a #30-mesh (600 μ) screen before weighing. One 500g sample of the dry powder formulation containing all of the ingredients listed immediately above was weighed and blended for 5 minutes at 2000 rpm in a Robot Coupe® high-shear granulator until adequately mixed to form a blended mass.
The blended mass was then processed as follows by wet-mass granulation/spheronization. Water was added to the dry powder formulation in a KitchenAid® KSM90 mixer (St. Joseph, MI) until the formulation was wet enough to be extruded through an LCI Benchtop Granulator (Tokyo, Japan) equipped with a 1.0 mm screen. The granules were allowed to dry at room temperature for 30 minutes before being spheronized in a Cleva™ 120 Spheronizer (Dorset, England) for 2.5 minutes. The wet-mass extruded pellets were dusted with microcrystalline cellulose during the
spheronization process to prevent agglomeration. The spherical pellets were dried for 12 hours at room temperature. Additionally, the pellets were not dried at an elevated temperature to prevent potential bead agglomeration, since Eudragit® 4135 F has a low glass transition temperature when plasticized. The dry pellets were sieved, and the 16-14 mesh size (1.18-1 ,40mm) pellets were saved for comparison testing.
EXAMPLE 2
The following process was used to make beads according to the hot-melt extrusion process of the invention. A blended mass was prepared according to Example 1. A 500 g sample of the blended mass was extraded using a multi-zone Randcastle Microtrader® RCP-0750 (Cedar Grove, NJ). The extrader temperature controllers were set as follows: zone 1 - 82.2°C, zone 2 = 118.3°C, zone 3 = 121.1°C, and die = 121.1°C. The formulation was fed into the hopper after the zones and die had equilibrated to the set temperatures. A cylindrical die 1.2mm in diameter was used, and the screw speed was 0.8-1.2 rpm. After exiting the die, the warm polymer strand with a diameter of 1.22 + 0.03 mm was fed into a Randcastle Pelletizer RCP-2.0 (epm 3.8 (speed of cylinders that feed extradate to the rotating knife); rpm 48 (rotational speed of the rotating knife)) and was cut into uniform pellets 1.22 ± 0.04 mm in length. The pellets were cooled to room temperature, and a 75 g sample was transferred into a Cleva 120 Spheronizer. A Milwaukee™ model 1220 (International Tool Corporation; Davie, FL) heat gun was used to blow hot air through the pellet exit of the spheronizer. The heat gun was used only as needed to soften the polymer pellets so that they would deform. The pellets were spheronized for approximately 0.75 - 1.5 hours at 65° C, and then they were cooled to room temperature and sieved. The pellets were dusted during spheronization. The 16-14 size (1.18-1.40mm) pellets were saved for comparison testing. See FIG. 2 for dissolution data.
EXAMPLE 3
The surface and internal morphology of the spherical beads produced according to Examples 1 and 2 were evaluated using a Hitachi® S-4500 field emission scanning electron microscope (Rolling Meadows, IL). Whole beads were viewed under 50X magnification whereas bead cross-sections were viewed under 10,000X magnification.
EXAMPLE 4
The particle size distribution of beads made according to Examples 1 and 2 was determined by sieving the beads through a series of U.S.A standard testing sieves (Arthur
H. Thomas Company; Philadelphia, PA) haying the following mesh sizes: 12, 14, 16, 18, 20, and 30. The same amount of beads produced by each process was used. The amount of beads retained by each sieve was then recorded.
EXAMPLE 5
The dissolution or release profile for beads made according to Examples 1 and 2 were determined as follows. Method A.
An USP 24 apparatus 2 dissolution testing paddle method was performed in a
VanKel® VK6010 (Gary, NC dissolution apparatus using 900ml of media at 37°C and
100 revolutions per minute (rpm). The pH 1.2 media was 0.1N HCL, and the pH 3.0, 6.8, and 7.4 media were phosphate buffered solutions. A VanKel® VK8000 auto sampler was used to withdraw 4ml of dissolution media at 0.25, 0.5, 1, 2, 4, 6, and 12 hour time points for each batch of beads. Dissolution media samples were diluted with dissolution media as needed and then assayed by spectrophotometry (DU-65, Beckman Instruments; Fullerton, CA) at a wavelength of 272 nm. Percent drag released was calculated based on an infinity concentration that was attained by mixing thoroughly the contents of individual dissolution vessels with a Polytron® (Brinkmann Instruments™; Westbury, NY).
Method B.
An USP 24 apparatus 3 dissolution testing reciprocating cylinder method was performed in a VanKel® Biodis™ II using 250ml of media at 37°C and 20 dips per minute (dpm). Stock solutions of the same media employed in Method A were used here. The bead samples were exposed serially to the media as follows: 2 hours in pH 1.2 medium; 1 hour in each of pH 3.0, 5.0, and 6.8 media; and 3 hours in pH 7.4 medium. Dissolution media was sample periodically and the samples were diluted with additional dissolution media as needed and then assayed by spectrophotometry at a wavelength of 272nm. All dissolution tests, USP apparatus 2 and 3, were performed in triplicate.
EXAMPLE 6
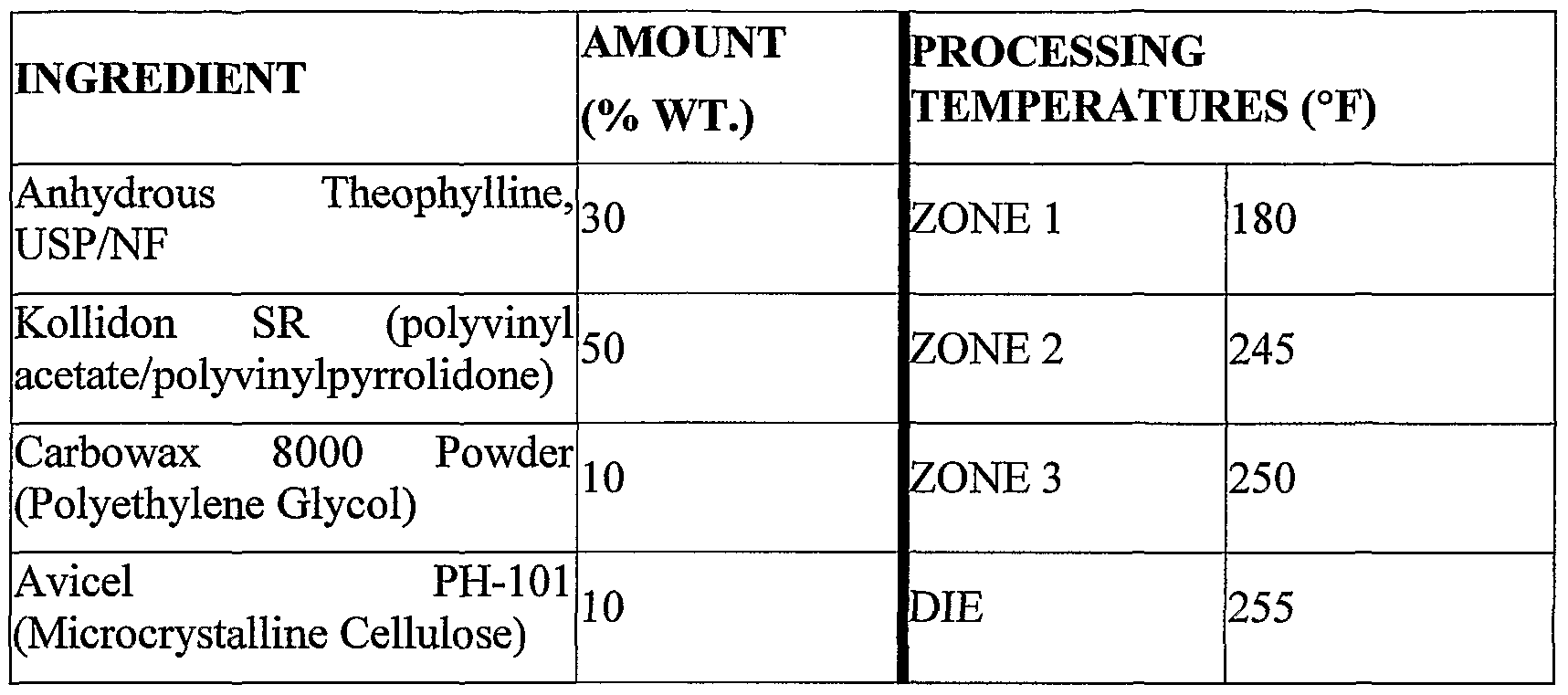
Various different hot-melt extraded/spheronized particles were made according to the invention using the following ingredients present in the amounts indicated. A multi- zone extruder was used and the temperature of the respective zones were set as indicated below.
A.
EXAMPLE 7
The following process was used to make beads according to the hot-melt extrasion process of the invention. A blended mass containing the following ingredients in the amounts indicated was prepared.
A 500 g sample of the blended mass was extraded using a multi-zone Randcastle Microtrader® RCP-0750 (Cedar Grove, NJ). The extrader temperature controllers were set as indicated above. The formulation was fed into the hopper after the zones and die had equilibrated to the set temperatures. A cylindrical die 1.2mm in diameter was used, and the screw speed was 0.8-1.2 rpm. After exiting the die, the polymer strand, having a diameter of 1.22 ± 0.03 mm, was cooled to 25 C, fed into a Randcastle Pelletizer RCP-2.0 (epm 2.9 (speed of cylinders that feed extradate to the rotating knife); rpm 55 (rotational speed of the rotating knife)) and cut into uniform pellets 1.22 ± 0.04 mm in length. The
pellets were cooled to room temperature, and a 75g sample was transferred into a Cleva 120 Spheronizer. A Milwaukee™ model 1220 (International Tool Corporation; Davie, FL) heat gun was used to blow hot air through the pellet exit of the spheronizer to heat the port to about 55° C. The heat gun was used only as needed to soften the polymer pellets so that they would deform. The pellets were spheronized for approximately 2.0 hours, and then they were cooled to room temperature, dusted with magnesium stearate and sieved. The 16-14 size (1.56 + 0.77 mm) pellets were saved for comparison testing. See FIG. 11 for dissolution data.
EXAMPLE 8
The following procedure was used to coat hot-melt extraded/spheronized beads prepared according to the invention. The coating formulation described below further controls the release of drug from the bead. The coating formulation contained the ingredients listed below in the amounts indicated.
The coating formulation was applied to the beads in a fluidized bed apparatus using the following parameters:
Batch Weight (beads) 300 g Inlet Temperature 30-33°C Outlet Temperature 25-27°C Nozzle Diameter 1.0 mm Spray Rate 1.8-2.0 g/min Curing Time 12 hrs at 37°C
EXAMPLE 9
The following examples can be made according to the procedure of Example 2. The listed ingredients can be used in the approximate amounts indicated and the extrasion can be conducted in a multi-zone extrader using the approximate temperatures indicated.
A.
B.
C.
D.
F.
G.
EXAMPLE 10
Spherical pellet porosity measurements were calculated using the following formula for percent porosity:
%P = [(dt- db) / db] x l00 where dt = true density and db = bulk density. The true density of the powder formulation was determined in triplicate using helium pycnometry to measure the density of the powder formulation prior to processing (Micrometrics® AccuPyc 1330 Pycnometer; Norcross, GA). Mercury porosymetry was employed to determine bulk
densities of spherical pellets, in triplicate, after processing (Micrometrics® PoreSizer 9320; Norcross, GA). Water was removed from powders and pellets by lyophilization for 72 hours prior to density determinations (Freeze Dryer 5; Kansas City, MO).
EXAMPLE 11
The following pellets were made according to the procedure of Example 2 except that the pelletizer parameters were as follows: epm 4.0; rpm 51. Also, the active agent in this example is diltiazem hydrochloride. See FIGS. 13 and 14 for dissolution data.
EXAMPLE 12
The following pellets were made according to the procedure of Example 6 except that the following ingredients and parameters were used.
The die size was 1.2mm and the screw speed was 1.2-1.4 rpm. The following parameters were used for the pelletizer: epm 8.9; rpm 120. See FIG. 15 for dissolution data.
EXAMPLE 13
The following pellets were made according to the procedure of Example 6 except that the following ingredients and parameters were used.
The die size was 1.2mm and the screw speed was 1.2-1.4 rpm. The following parameters were used for the pelletizer: epm 9.5; rpm 130. See FIG. 16 for dissolution data.
The above is a detailed description of particular embodiments of the invention. It will be appreciated that, although specific embodiments of the invention have been described herein for purposes of illustration, various modifications may be made without departing from the spirit and scope of the invention. Accordingly, the invention is not limited except as by the appended claims. All of the embodiments disclosed and claimed herein can be made and executed without undue experimentation in light of the present disclosure.